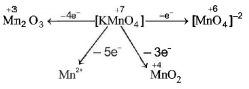
251. When $$KMn{O_4}$$ acts as an oxidising agent and ultimately forms $${\left[ {Mn{O_4}} \right]^{ - 2}},Mn{O_2},M{n_2}{O_3},M{n^{ + 2}}$$ then the number of electrons transfered in each case respectively is
A
$$4,3,1,5$$
B
$$1,5,3,7$$
C
$$1,3,4,5$$
D
$$3,5,7,1.$$
Answer :
$$1,3,4,5$$
252. How many unpaired electrons are present in $$N{i^{2 + }}$$ ?
A
$$0$$
B
$$2$$
C
$$4$$
D
$$8$$
Answer :
$$2$$
253. The melting points of $$Cu,Ag$$ and $$Au$$ follow the order
A
$$Cu > Ag > Au$$
B
$$Cu > Au > Ag$$
C
$$Au > Ag > Cu$$
D
$$Ag > Au > Cu$$
Answer :
$$Cu > Au > Ag$$
254. Which one of the following nitrates will leave behind a metal on strong heating?
A
Copper nitrate
B
Manganese nitrate
C
Silver nitrate
D
Ferric nitrate
Answer :
Silver nitrate
255. Identify the incorrect statement among the following.
A
$$4f$$ and $$5f$$ orbitals are equally shielded.
B
$$d$$ - Block elements show irregular and erratic chemical properties among themselves.
C
$$La$$ and $$Lu$$ have partially filled $$d$$ - orbitals and no other partially filled orbitals.
D
The chemistry of various lanthanoids is very similar.
Answer :
$$4f$$ and $$5f$$ orbitals are equally shielded.
256. The trend of basicity of lanthanoid hydroxides
A
increases across the lanthanoid series
B
decreases across the lanthanoid series
C
first increases and then decreases
D
first decreases and then increases
Answer :
decreases across the lanthanoid series
257.
Which one of the following ions is the most stable in aqueous solution?
$$\left( {{\text{At}}{\text{. no}}{\text{.}}\,Ti = 22,V = 23,} \right.$$ $$\left. {Cr = 24,Mn = 25} \right)$$
A
$$C{r^{3 + }}$$
B
$${V^{3 + }}$$
C
$$T{i^{3 + }}$$
D
$$M{n^{3 + }}$$
Answer :
$$M{n^{3 + }}$$
258. In acidic medium, $$KMn{O_4}$$ oxidises $$FeS{O_4}$$ solution. Which of the following statements is correct?
A
$$10\,mL$$ of $$1\,N\,KMn{O_4}$$ solution oxidises $$10\,mL$$ of $$5\,N\,FeS{O_4}$$ solution.
B
$$10\,mL$$ of $$1\,M\,KMn{O_4}$$ solution oxidises $$10\,mL$$ of $$5\,M\,FeS{O_4}$$ solution.
C
$$10\,mL$$ of $$1\,M\,KMn{O_4}$$ solution oxidises $$10\,mL$$ of $$1\,M\,FeS{O_4}$$ solution.
D
$$10\,mL$$ of $$1\,N\,KMn{O_4}$$ solution oxidises $$10\,mL$$ of $$0.1\,M\,FeS{O_4}$$ solution.
Answer :
$$10\,mL$$ of $$1\,M\,KMn{O_4}$$ solution oxidises $$10\,mL$$ of $$5\,M\,FeS{O_4}$$ solution.
259. Interstitial compounds are
A
non-stoichiometric and are ionic in nature
B
non-stoichiometric and are covalent in nature
C
non-stoichiometric and are neither typically ionic nor covalent in nature
D
stoichiometric and are neither ionic nor covalent in nature
Answer :
non-stoichiometric and are neither typically ionic nor covalent in nature
260. Which compound does not dissolve in hot, dilute $$HN{O_3}$$ ?
A
$$HgS$$
B
$$PbS$$
C
$$CuS$$
D
$$CdS$$
Answer :
$$HgS$$
 It has $$2$$ unpaired electrons.
It has $$2$$ unpaired electrons.