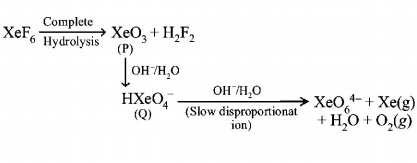221.
Fill in the blanks by choosing the appropriate option. $$Conc.\,{H_2}S{O_4}$$ chars paper, wood and sugar by removing $$\underline {\left( {\text{i}} \right)} $$ from them. It is also known as $$\underline {\left( {{\text{ii}}} \right)} .$$ It is manufactured by $$\underline {\left( {{\text{iii}}} \right)} $$ process. It is a strong $$\underline {\left( {{\text{iv}}} \right)} $$ and $$\underline {\left( {\text{v}} \right)} $$ acid.
| (i) | (ii) | (iii) | (iv) | (v) | |
|---|---|---|---|---|---|
| (a) | $${H_2}O$$ | oil of vitriol | Contact | Oxidising | Dibasic |
| (b) | $${O_2}$$ | oil of vitriol | oleum | dehydrating | monobasic |
| (c) | $${H_2}O$$ | oil of olay | Solvay | dehydrating | dibasic |
| (d) | $$S{O_2}$$ | oil of winter green | contact | oxidising | monobasic |
A
(a)
B
(b)
C
(c)
D
(d)
Answer :
(a)
222. Silicon is an important constituent of
A
sand
B
atmosphere
C
plants
D
water bodies
Answer :
sand
223.
Which is the best description of behaviour of bromine in the reaction given below?
$${H_2}O + B{r_2} \to HBr + HOBr$$
A
Only oxidised
B
Only reduced
C
Both oxidised and reduced
D
Only proton accepted
Answer :
Both oxidised and reduced
224. $$BC{l_3}$$ is a planar molecule whereas $$NC{l_3}$$ is pyramidal because
A
$$BC{l_3}$$ has no lone pair of electrons but $$NC{l_3}$$ has a lone pair of electrons.
B
$$B - Cl$$ bond is more polar than $$N - Cl$$ bond.
C
nitrogen atom is smaller than boron atom.
D
$$N - Cl$$ bond is more covalent than $$B - Cl$$ bond.
Answer :
$$BC{l_3}$$ has no lone pair of electrons but $$NC{l_3}$$ has a lone pair of electrons.
225. The exhibition of highest co-ordination number depends on the availability of vacant orbitals in the central atom. Which of the following elements is not likely to act as central atom in $$MF_6^{3 - }?$$
A
$$B$$
B
$$Al$$
C
$$Ga$$
D
$$In$$
Answer :
$$B$$
226.
Under ambient conditions, the total number of gases released
as products in the final step of the reaction scheme shown
below is
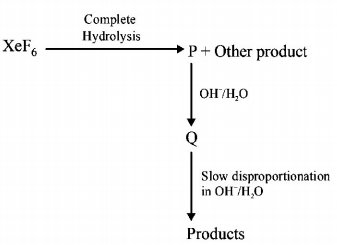
A
$$0$$
B
$$1$$
C
$$2$$
D
$$3$$
Answer :
$$2$$
227. On heating, lead nitrate forms oxides of nitrogen and lead. The oxides formed are _________.
A
$${N_2}O,PbO$$
B
$$N{O_2},PbO$$
C
$$NO,PbO$$
D
$$NO,Pb{O_2}$$
Answer :
$$N{O_2},PbO$$
228. $$Conc.\,HN{O_3}$$ is heated with $${P_2}{O_5}$$ to form :
A
$$N{O_2}$$
B
$$NO$$
C
$${N_2}{O_5}$$
D
$${N_2}O$$
Answer :
$${N_2}{O_5}$$
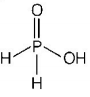
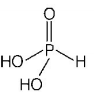
229. Which is the correct statement for the given acids?
A
Phosphinic acid is a monoprotic acid while phosphonic acid is a diprotic acid
B
Prosphinic acid is a diprotic acid while phosphonic acid is a monoprotic acid
C
Both are triprotic acids
D
Both are diprotic acids
Answer :
Phosphinic acid is a monoprotic acid while phosphonic acid is a diprotic acid
230. The acid having $$\,O - O\,$$ bond is
A
$${H_2}{S_2}{O_3}$$
B
$${H_2}{S_2}{O_6}$$
C
$${H_2}{S_2}{O_8}$$
D
$${H_2}{S_4}{O_6}$$
Answer :
$${H_2}{S_2}{O_8}$$