1. Reagent used to convert allyl alcohol to acrolein is
A
$$Mn{O_2}$$
B
$${H_2}{O_2}$$
C
$$Os{O_4}$$
D
$$KMn{O_4}$$
Answer :
$$Mn{O_2}$$
2. What would be the reactant and reagent used to obtain 2, 4-dimethylpentan-3-ol?
A
Propanal and propyl magnesium bromide
B
3-Methylbutanal and 2-methyl magnesium iodide
C
2, 2-Dimethylpropanone and methyl magnesium iodide
D
2-Methylpropanal and isopropyl magnesium iodide
Answer :
2-Methylpropanal and isopropyl magnesium iodide
3. Tertiary butyl alcohol can be prepared by the reaction of
A
acetaldehyde and ethyl magnesium iodide
B
acetone and methyl magnesium iodide
C
formaldehyde and propyl magnesium iodide
D
butanone and methyl magnesium iodide
Answer :
acetone and methyl magnesium iodide
4. Which of the following alcohols gives the best yield of dialkyl ether on being heated with a trace of sulphuric acid?
A
2-Pentanol
B
2-Methyl-2-butanol
C
1-Pentanol
D
2-Propanol
Answer :
1-Pentanol
5. From amongst the following alcohols the one that would react fastest with conc. $$HCl$$ and anhydrous $$ZnC{l_2},$$ is
A
2 - Butanol
B
2 - Methylpropan - 2 - $$ol$$
C
2 - Methylpropanol
D
1 - Butanol
Answer :
2 - Methylpropan - 2 - $$ol$$
6.
A primary alcohol, $${C_3}{H_8}O\left( A \right)$$ on heating with sulphuric acid undergo dehydration to give an alkene, $$B.$$ $$B$$ when reacted with $$HCl$$ gave $$C,$$ which on treatment with aqueous $$KOH$$ gives compound $$D\left( {{C_3}{H_8}O} \right).$$
$$A$$ and $$D$$ are
A
functional isomers
B
position isomers
C
chain isomers
D
stereoisomers
Answer :
position isomers
7. Which of the following compounds is a polyhydric alcohol?
A
$${C_2}{H_6}O$$
B
$${C_2}{H_4}O$$
C
$${C_3}{H_8}{O_3}$$
D
$${C_4}{H_{10}}O$$
Answer :
$${C_3}{H_8}{O_3}$$
8. An alcohol $$X$$ when treated with hot conc. $${H_2}S{O_4}$$ gave an alkene $$ Y$$ with formula $${C_4}{H_8}.$$ This alkene on ozonolysis gives single product with molecular formula $${C_2}{H_4}O.$$ The alcohol is
A
butan-1-ol
B
butan-2-ol
C
2-methylpropan-1-ol
D
2, 2-dimethylbutan-1-ol
Answer :
butan-2-ol
9. Which of the following synthesis gives 3-methyl-1- hexanol ?
A
2 - bromohexane \[\xrightarrow[{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}]{Mg}\,\,\,\xrightarrow[\left( ii \right)\,{{H}_{3}}{{O}^{+}}]{\left( i \right)\,{{H}_{2}}C=O}\]
B
2 - bromopentane \[\xrightarrow[{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}]{Mg}\] 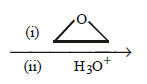
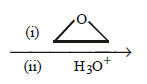
C
3 - bromopentane \[\xrightarrow[{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}]{Mg}\,\,\,\xrightarrow[\left( ii \right)\,{{H}_{3}}{{O}^{+}}]{\left( i \right)\,C{{H}_{3}}CH=O}\]
D
1 - bromobutane \[\xrightarrow[{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}]{Mg}\,\,\,\xrightarrow[\left( ii \right)\,{{H}_{3}}{{O}^{+}}]{\left( i \right)\,C{{H}_{3}}COC{{H}_{3}}}\]
Answer :
2 - bromopentane \[\xrightarrow[{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}]{Mg}\] 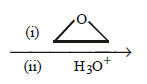
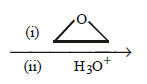
10. If a particular ether contains $$60\% $$ carbon and $$13.3\% $$ hydrogen, its formula could be
A
$${C_2}{H_6}O$$
B
$${C_4}{H_{10}}O$$
C
$${C_5}{H_{12}}O$$
D
$${C_3}{H_8}O$$
Answer :
$${C_3}{H_8}O$$



