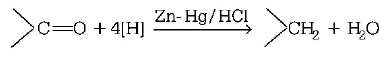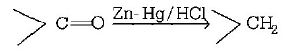151. The final product $$(Y)$$ in the following sequence of chemical reaction is \[C{{H}_{3}}OH\xrightarrow[{{300}^{\circ }}C]{Cu}X\xrightarrow{NaOH}\] \[Y+C{{H}_{3}}OH\]
A
an alkene
B
a carboxylic acid
C
an aldehyde
D
sodium salt of carboxylic acid
Answer :
sodium salt of carboxylic acid
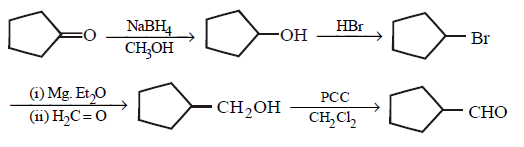
152.
What is $$D$$ in the following sequence of reactions ?

\[\xrightarrow[C{{H}_{3}}OH]{NaB{{H}_{4}}}A\xrightarrow{HBr}B\xrightarrow[\begin{smallmatrix}
\left( ii \right)\,{{H}_{2}}C=O \\
\left( iii \right)\,{{H}_{3}}{{O}^{+}}
\end{smallmatrix}]{\left( i \right)\,Mg,E{{t}_{2}}O}C\xrightarrow[C{{H}_{2}}C{{l}_{2}}]{PCC}D\]
A
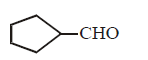

B


C
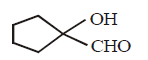
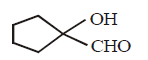
D
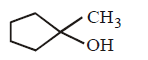

Answer :


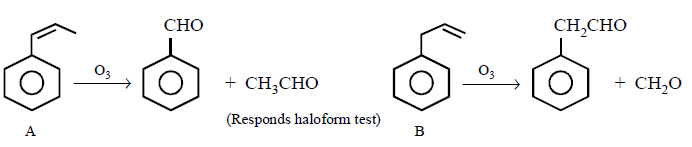
153.
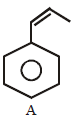 \[\xrightarrow{\text{Ozonolysis}}X+Y\]
\[\xrightarrow{\text{Ozonolysis}}X+Y\]
 \[\xrightarrow{\text{Ozonolysis}}C+D\]
\[\xrightarrow{\text{Ozonolysis}}C+D\]
From the ozonolysis products, the two isomers $$A$$ and $$B$$ can be distinguished with the help of
A
Fehling solution
B
Tollen’s reagent
C
Haloform test
D
only spectroscopy
Answer :
Haloform test
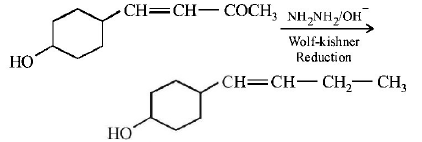
154.
In the given transformation, which of the following is the most appropriate reagent ?

A
$$N{H_2}N{H_2}{\text{,}}\,\mathop O\limits^\Theta H$$
B
$$Zn - Hg/HCl$$
C
$$Na,Liq\,N{H_3}$$
D
$$NaB{H_4}$$
Answer :
$$N{H_2}N{H_2}{\text{,}}\,\mathop O\limits^\Theta H$$
155. $${\left( {C{H_3}} \right)_3}C - CHO$$ does not undergo aldol condensation due to
A
three electron donating methyl groups
B
cleavage taking place between $$ - C - CHO$$ bond
C
absence of alpha hydrogen atom in the molecule
D
bulky $${\left( {C{H_3}} \right)_3}C - $$ group
Answer :
absence of alpha hydrogen atom in the molecule
156. Reduction of aldehydes and ketones into hydrocarbons using zinc amalgam and $$conc.$$ $$HCl$$ is called
A
Clemmensen reduction
B
Cope reduction
C
Dow reduction
D
Wolff-Kishner reduction
Answer :
Clemmensen reduction
157. Which of the following carbonyl compounds is most polar?
A
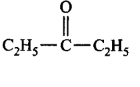
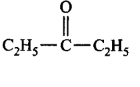
B


C


D
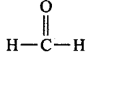
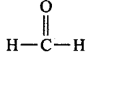
Answer :
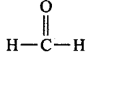
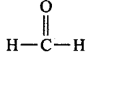
158. Which is not true about acetophenone ?
A
It reacts with 2, 4 - dinitrophenylhydrazine to form 2, 4 - dinitrophenylhydrazone
B
It reacts with Tollen’s reagent to form silver mirror
C
It reacts with $${I_2}/NaOH$$ to form iodoform
D
On oxidation with alkaline $$KMn{O_4}$$ followed by hydrolysis it gives benzoic acid
Answer :
It reacts with Tollen’s reagent to form silver mirror
159.
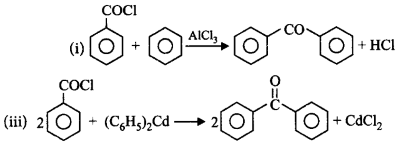
Which of the following reactions will give benzophenone?
(i) Benzoyl chloride $$+$$ Benzene $$+$$ $$AlC{l_3}$$
(ii) Benzoyl chloride $$+$$ Phenylmagnesium bromide
(iii) Benzoyl chloride $$+$$ Diphenyl cadmium
A
(i) and (ii)
B
(ii) and (iii)
C
(i) and (iii)
D
(i), (ii) and (iii)
Answer :
(i) and (iii)
160. Clemmensen reduction of a ketone is carried out in the presence of which of the following?
A
$$Zn - Hg\,$$ with $$HCl$$
B
$$LIAI{H_4}$$
C
$${H_2}$$ and $$Pt$$ as catalyst
D
Glycol with $$KOH$$
Answer :
$$Zn - Hg\,$$ with $$HCl$$
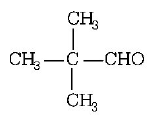 does not undergo aldol condensation because it does not contain $$\alpha $$ - hydrogen atom.
does not undergo aldol condensation because it does not contain $$\alpha $$ - hydrogen atom.