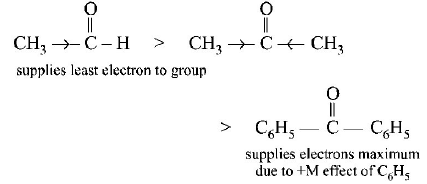
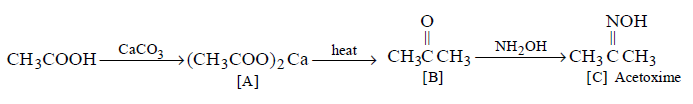211.
Match the column I with column II and mark the appropriate choice.
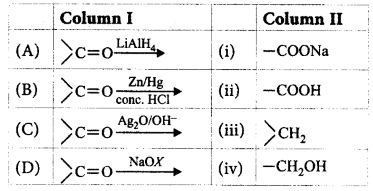
A
A - i, B - ii, C - iii, D - iv
B
A - iv, B - iii, C - ii, D - i
C
A - ii, B - iv, C - iii, D - i
D
A - iii, B - i, C - ii, D - iv
Answer :
A - iv, B - iii, C - ii, D - i
212. Choose the correct statement regarding the physical properties of carbonyl compound.
A
All aldehydes are insoluble in benzene.
B
Higher aldehydes are more fragrant.
C
$$n$$-Butane has more boiling point than acetone.
D
Methanal and propanone are immiscible with water in all proportions.
Answer :
Higher aldehydes are more fragrant.
213. Cannizaro’s reaction is not given by :
A
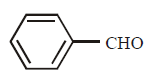

B
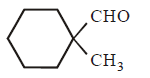
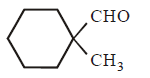
C
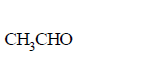
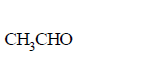
D


Answer :
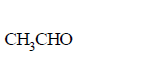
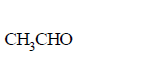
214. Aldehydes and ketones will not form crystalline derivatives with
A
sodium bisulphite
B
phenyl hydrazine
C
semicarbazide hydrochloride
D
dihydrogen sodiurn phosphate
Answer :
dihydrogen sodiurn phosphate
215. Which of the following statements is correct?
A
$$CC{l_3} - CHO$$ gives aldol condensation.
B
When mixture of ethanal and propanal is treated with aqueous $$NaOH,$$ the product contains four aldols.
C
Mixture of $$HCHO$$ and $$C{H_3}CHO$$ will not give aldol condensation.
D
$$HCHO$$ is least reactive towards oxidation.
Answer :
When mixture of ethanal and propanal is treated with aqueous $$NaOH,$$ the product contains four aldols.
216. The Cannizzaro reaction is not given by
A
trimethylacetaldehye
B
acetaldehyde
C
benzaldehyde
D
formaldehyde
Answer :
acetaldehyde
217. An organic compound $$'A'$$ has the molecular formula \[{{C}_{3}}{{H}_{6}}O.\] It undergoes iodoform test. When staturated with $$HCl$$ it gives $$'B'$$ of molecular formula \[{{C}_{9}}{{H}_{14}}O.\] $$'A'$$ and $$'B'$$ respectively are
A
Propanal and mesitylene
B
Propanone and mesityl oxide
C
Propanone and 2, 6 - dimethyl - 2, 5 - heptadien - 4 - one
D
Propanone and mesitylene oxide
Answer :
Propanone and mesitylene oxide
218. A substance $${C_4}{H_{10}}O$$ yields on oxidation a compound $${C_4}{H_8}O$$ which gives an oxime and a positive iodoform test. The original substance on treatment with conc. $${H_2}S{O_4}$$ gives $${C_4}{H_8}.$$ The structure of the compound is
A
$$C{H_3}C{H_2}C{H_2}C{H_2}OH$$
B
$$C{H_3}CH\left( {OH} \right)C{H_2}C{H_3}$$
C
$${\left( {C{H_3}} \right)_3}COH$$
D
$$C{H_3}C{H_2} - O - C{H_2}C{H_3}$$
Answer :
$$C{H_3}CH\left( {OH} \right)C{H_2}C{H_3}$$
219. Which of the following does not answer iodoform test?
A
$$n$$ - Butyl alcohol
B
$$sec$$ - Butyl alcohol
C
Acetophenone
D
Acetaldehyde
Answer :
$$n$$ - Butyl alcohol
220.
The correct order of reactivity of \[PhMgBr\] with
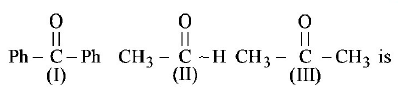
A
(I) > (II) > (III)
B
(III) > (II) > (I)
C
(II) > (III) > (I)
D
(I) > (III) > (II)
Answer :
(II) > (III) > (I)