1.
$$C{H_2}$$  \[C{{H}_{2}}+HBr\xrightarrow{\text{peroxide}}A,\] then $$A$$ is
\[C{{H}_{2}}+HBr\xrightarrow{\text{peroxide}}A,\] then $$A$$ is
A


B


C

D


Answer :


2. The negative part of the addendum ( the molecule to be added ) adds on to the carbon atom of the double bond containing the least number of hydrogen atoms. This rule is known as
A
Saytzeff's rule
B
Peroxide rule
C
Markovnikov's rule
D
Hoffmann rule
Answer :
Markovnikov's rule
3.
Classify the following compounds as primary, secondary and tertiary halides.
(i) 1-Bromobut-2-ene
(ii) 4-Bromopent-2-ene
(iii) 2-Bromo-2-methylpropane
A
(i) - secondary, (ii) - tertiary, (iii) - primary
B
(i) - secondary, (ii) - primary, (iii) - tertiary
C
(i) - primary, (ii) - tertiary, (iii) - secondary
D
(i) - primary, (ii) - secondary, (iii) - tertiary
Answer :
(i) - primary, (ii) - secondary, (iii) - tertiary
4.
Identify the products $$(A)$$ and $$(B)$$ in the reactions.
$$\eqalign{
& RX + AgCN \to \underline {\,\,\left( A \right)\,\,} + AgX \cr
& RX + KCN \to \underline {\,\,\left( B \right)\,\,} + KX \cr} $$
A
$$\left( A \right){\text{ - }}RCN,\,\left( B \right){\text{ - }}RCN$$
B
$$\left( A \right){\text{ - }}RCN,\,\left( B \right){\text{ - }}RNC$$
C
$$\left( A \right){\text{ - }}RNC,\,\left( B \right){\text{ - }}RCN$$
D
$$\left( A \right){\text{ - }}RNC,\,\left( B \right){\text{ - }}RNC$$
Answer :
$$\left( A \right){\text{ - }}RNC,\,\left( B \right){\text{ - }}RCN$$
5.
 \[\xrightarrow[280\,K]{NaN{{O}_{2}}+HCl}K\xrightarrow{KI}Y\]
\[\xrightarrow[280\,K]{NaN{{O}_{2}}+HCl}K\xrightarrow{KI}Y\]
$$X$$ and $$Y$$ in the reaction are
A


B


C


D


Answer :


6. The position of $$ - Br$$ in the compound $$C{H_3}CH = CHC\left( {Br} \right){\left( {C{H_3}} \right)_2}$$ can be classified as _________.
A
allyl
B
aryl
C
vinyl
D
secondary
Answer :
allyl
7.
In the following reaction sequence :
\[\underset{\left( {{C}_{3}}{{H}_{6}}C{{l}_{2}} \right)}{\mathop{I}}\,\xrightarrow{KOH\left( aq \right)}II\xrightarrow[\left( ii \right)\,{{H}_{2}}O/{{H}^{+}}]{\left( i \right)\,C{{H}_{3}}MgBr}III\xrightarrow{Anhy.ZnC{{l}_{2}}+Conc.HCl}\text{given}\,\text{turbidity immediately}\]
The compound $$I$$ is :
A


B


C


D


Answer :


8. Which alkyl halide exhibits complete racemisation in $${S_N}1$$ reaction?
A
$${\left( {C{H_3}} \right)_2}CHCl$$
B
$$C{H_3}C{H_2}C{H_2}Cl$$
C
$$C{H_3}C{H_2}Cl$$
D
$${C_6}{H_5}C{H_2}Cl$$
Answer :
$${C_6}{H_5}C{H_2}Cl$$
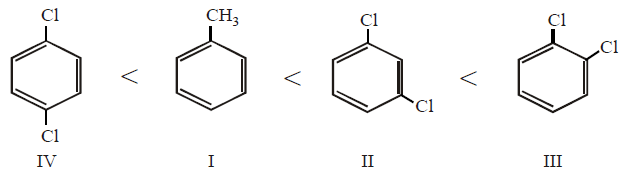
9.
Arrange the following compounds in order of increasing dipole moment :
(I) Toluene
(II) $$m$$ - dichlorobenzene
(III) $$o$$ - dichlorobenzene
(IV) $$p$$ - dichlorobenzene
A
I < IV < II < III
B
IV < I < II < III
C
IV < I < III < II
D
IV < II < I < III
Answer :
IV < I < II < III
10. A primary alkyl halide would prefer to undergo __________.
A
$${S_N}1$$ reaction
B
$${S_N}2$$ reaction
C
$$\alpha $$ - elimination
D
racemisation
Answer :
$${S_N}2$$ reaction