91. In $$C{H_3}C{H_2}OH,$$ the bond that undergoes heterolytic cleavage most readily is
A
$$C - C$$
B
$$C - O$$
C
$$C - H$$
D
$$O - H$$
Answer :
$$O - H$$
92. In the compound $$C{H_2} = CH - C{H_2} - C{H_2} - C \equiv $$ $$CH,$$ the $${C_2} - {C_3}$$ bond is of the type,
A
$$sp - s{p^2}$$
B
$$s{p^3} - s{p^3}$$
C
$$sp - s{p^3}$$
D
$$s{p^2} - s{p^3}$$
Answer :
$$s{p^2} - s{p^3}$$
93. The reaction : \[{{\left( C{{H}_{3}} \right)}_{3}}C-Br\xrightarrow{{{H}_{2}}O}{{\left( C{{H}_{3}} \right)}_{3}}C-OH\]
A
elimination reaction
B
substitution reaction
C
free radical reaction
D
displacement reaction.
Answer :
substitution reaction
94.
The $$IUPAC$$ name of the following compound is
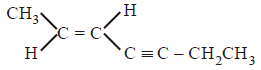
A
$$(E){\text{ - 2 - hepten - 4 - yne}}$$
B
$$(Z){\text{ - 5 - hepten - 3 - yne}}$$
C
$$(E){\text{ - 5 - hepten - 3 - yne}}$$
D
$$(Z){\text{ - 2 - hepten - 4 - yne}}$$
Answer :
$$(E){\text{ - 2 - hepten - 4 - yne}}$$
95. In the anion $$HCO{O^ - }$$ the two carbon-oxygen bonds are found to be of equal length. what is the reason for it ?
A
The $$C = O$$ bond is weaker than the $$C - O$$ bond
B
The anion $$HCO{O^ - }$$ has two resonating structures
C
The anion is obtained by removal of a proton from the
acid molecule
D
Electronic orbitals of carbon atom are hybridised
Answer :
Electronic orbitals of carbon atom are hybridised
96. Which of the following compounds is not chiral ?
A
$$DC{H_2}C{H_2}C{H_2}Cl$$
B
$$C{H_3}C{H_2}CHDCl$$
C
$$C{H_3}CHDC{H_2}Cl$$
D
$$C{H_3}CHClC{H_2}D$$
Answer :
$$DC{H_2}C{H_2}C{H_2}Cl$$
97. Which molecule will be most reactive for $${S_N}1$$ reaction ?
A
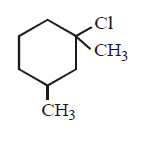

B


C
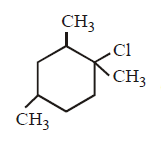

D


Answer :


98. $$0.2\,g$$ of an organic compound contains $$C, H$$ and $$O.$$ On combustion, it yields $$0.15\,g\,C{O_2}$$ and $$0.12\,g$$ $${H_2}O.$$ The percentage of $$C, H$$ and $$O$$ respectively is
A
$$C = 15\% ,H = 20\% ,O = 65\% $$
B
$$C = 10\% ,H = 8.2\% ,O = 81.8\% $$
C
$$C = 12.2\% ,H = 8.8\% ,O = 79\% $$
D
$$C = 20\% ,H = 6.66\% ,O = 73.34\% $$
Answer :
$$C = 20\% ,H = 6.66\% ,O = 73.34\% $$
99.
$$IUPAC$$ name of the following is
$$C{H_2} = CH - C{H_2} - C{H_2} - C \equiv CH$$
A
$$1, 5-hexenyne$$
B
$$1-hexene-5-yne$$
C
$$1-hexyne-5-ene$$
D
$$1, 5-hexynene$$
Answer :
$$1-hexene-5-yne$$
100.
The IUPAC name of 
A
$$4 - hydroxy - 1 - methyl{\text{ }}pentanal$$
B
$$4 - hydroxy - 2 - methyl{\text{ }}pent - 2 - en - 1{\text{ }} - al$$
C
$$2 - hydroxy - 4 - methyl{\text{ }}pent - 3 - en - 5 - al$$
D
$$2 - hydroxy - 3 - methyl{\text{ }}pent - 2 - en - 5 - al$$
Answer :
$$4 - hydroxy - 2 - methyl{\text{ }}pent - 2 - en - 1{\text{ }} - al$$



