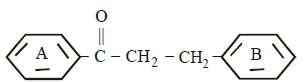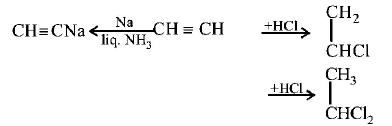1.
The major product obtained in the following reaction
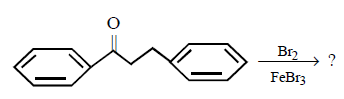
A
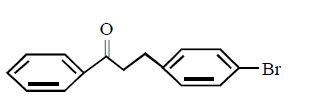

B
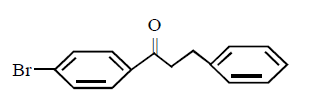

C
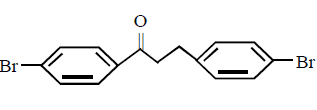

D
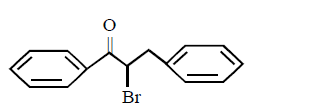

Answer :


2. Which of these will not react with acetylene ?
A
$$NaOH$$
B
ammonical $$AgN{O_3}$$
C
$$Na$$
D
$$HCl$$
Answer :
$$NaOH$$
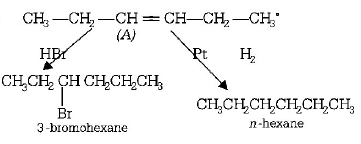
3. In the presence of platinum catalyst , hydrocarbon $$A$$ adds hydrogen to form $$n$$ - hexane. When hydrogen bromide is added to $$A$$ instead of hydrogen only a single bromo compound is formed. Which of the following is $$A?$$
A
$$C{H_3} - C{H_2} - CH = CH - C{H_2} - C{H_3}$$
B
$$C{H_3} - C{H_2} - C{H_2} - CH = CH - C{H_3}$$
C
$$C{H_3} - CH = CH - C{H_2} - C{H_2} - C{H_3}$$
D
$$C{H_2} = CH - C{H_2} - C{H_2} - C{H_2} - C{H_3}$$
Answer :
$$C{H_3} - C{H_2} - CH = CH - C{H_2} - C{H_3}$$
4.
The ozonolysis product$$(s)$$ of the following reaction is(are)
\[C{{H}_{3}}C{{H}_{2}}-C\equiv CH\xrightarrow[\left( \text{ii} \right)\,{{H}_{2}}O]{\left( \text{i} \right)\,{{O}_{3}}}\] \[\text{Product}\left( s \right)\]
A
$$C{H_3}COC{H_3}$$
B
$$C{H_3}COC{H_3} + HCHO$$
C
$$C{H_3}COOH + HCOOH$$
D
$$C{H_3}C{H_2}COOH + HCOOH$$
Answer :
$$C{H_3}C{H_2}COOH + HCOOH$$
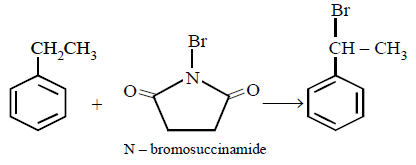
5. The product of the reaction between ethyl benzene and $$N$$ - bromosuccinamide is
A


B
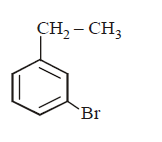

C
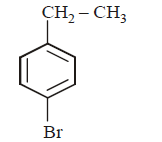

D
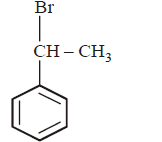

Answer :


6. Identify the reagent from the following list which can easily distinguish between 1 - butyne and 2 - butyne
A
bromine, $$CC{l_4}$$
B
$${H_2}$$ Lindlar catalyst
C
dilute $${H_2}S{O_4},HgS{O_4}$$
D
ammonical $$C{u_2}C{l_2}$$ solution
Answer :
ammonical $$C{u_2}C{l_2}$$ solution
7.
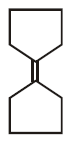 \[\xrightarrow[Zn]{{{O}_{3}}}A\xrightarrow[\text{Or}\,\,LiAl{{H}_{4}}]{{{H}_{3}}Ni}B\xrightarrow[\Delta ]{H+}\left( C \right);\] Product $$(C)$$ of the reaction is
\[\xrightarrow[Zn]{{{O}_{3}}}A\xrightarrow[\text{Or}\,\,LiAl{{H}_{4}}]{{{H}_{3}}Ni}B\xrightarrow[\Delta ]{H+}\left( C \right);\] Product $$(C)$$ of the reaction is
A
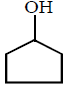

B


C


D


Answer :


8. Which of the following is an aromatic species ?
A


B
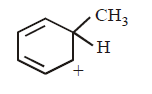

C


D


Answer :


9. \[Ph-C\equiv C-C{{H}_{3}}\xrightarrow{H{{g}^{2+}}/{{H}^{+}}}A.A\,\text{is}\,:\]
A
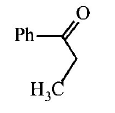

B
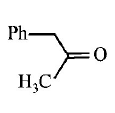

C


D


Answer :


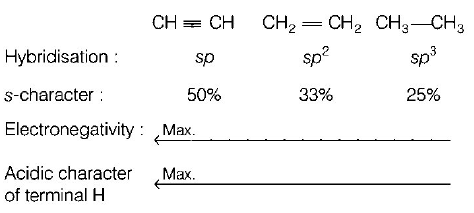
10. Which one is the correct order of acidity?
A
$$C{H_2} = C{H_2} > C{H_3} - CH = $$ $$C{H_2} > C{H_3} - C \equiv CH > CH \equiv CH$$
B
$$CH \equiv CH > C{H_3} - C \equiv $$ $$CH > C{H_2} = C{H_2} > C{H_3} - C{H_3}$$
C
$$CH \equiv CH > C{H_2} = $$ $$C{H_2} > C{H_3} - C \equiv CH > C{H_3} - C{H_3}$$
D
$$C{H_3} - C{H_3} > C{H_2} = $$ $$C{H_2} > C{H_3} - C \equiv CH > CH \equiv CH$$
Answer :
$$CH \equiv CH > C{H_3} - C \equiv $$ $$CH > C{H_2} = C{H_2} > C{H_3} - C{H_3}$$