11. Which of the following species does not show aromaticity?
A


B
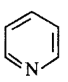

C
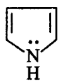

D
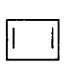

Answer :


12. Which of the following reactions will yield 2, 2 - dibromopropane?
A
$$C{H_3} - CH = C{H_2} + HBr \to $$
B
$$C{H_3} - C \equiv CH + 2HBr \to $$
C
$$C{H_3}CH = CHBr + HBr \to $$
D
$$CH \equiv CH + 2HBr \to $$
Answer :
$$C{H_3} - C \equiv CH + 2HBr \to $$
13.
What is the end product of the following sequences of operations ?
\[Ca{{C}_{2}}\xrightarrow{{{H}_{2}}O}A\xrightarrow[H{{g}^{2+}}]{dil.\,{{H}_{2}}S{{O}_{4}}}B\xrightarrow[{{H}_{2}}]{Ni}C\]
A
$${\text{Methyl alcohol}}$$
B
$${\text{Acetaldehyde}}$$
C
$${C_2}{H_5}OH$$
D
$${C_2}{H_4}$$
Answer :
$${C_2}{H_5}OH$$
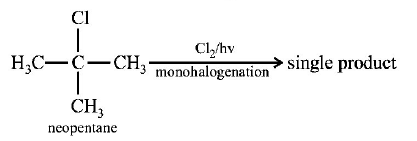
14. Which branched chain isomer of the hydrocarbon with molecular mass $$72u$$ gives only one isomer of mono substituted alkyl halide ?
A
Tertiary butyl chloride
B
Neopentane
C
Isohexane
D
Neohexane
Answer :
Neopentane
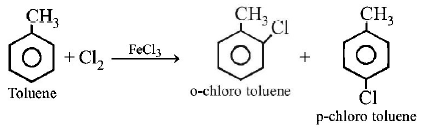
15. The reaction of toluene with $$C{l_2}$$ in presence of $$FeC{l_3}$$ gives predominantly
A
$$m$$ - chlorobenzene
B
benzoyl chloride
C
benzyl chloride
D
$$o$$ - and $$p$$ - chlorotoluene.
Answer :
$$o$$ - and $$p$$ - chlorotoluene.
16.

Hydrogenation of the above compound in the presence of
poisoned palladium catalyst gives
A
an optically active compound
B
an optically inactive compound
C
a racemic mixture
D
a diastereomeric mixture
Answer :
an optically inactive compound
17. The number of chain isomers possible for hydrocarbon $${C_5}{H_{12}}$$ is
A
3
B
5
C
4
D
6
Answer :
3
18. Marsh gas mainly contains
A
$${C_2}{H_2}$$
B
$$C{H_4}$$
C
$${H_2}S$$
D
$$CO$$
Answer :
$$C{H_4}$$
19. One mole of a symmetrical alkene on ozonolysis gives two moles of an aldehyde having a molecular mass of $$44u.$$ The alkene is
A
propene
B
1 - butene
C
2 - utene
D
ethene
Answer :
2 - utene
20. In the presence of peroxide, hydrogen chloride and hydrogen iodide do not give anti-Markovnikov addition to alkenes because
A
both are highly ionic
B
one is oxidizing and the other is reducing
C
one of the steps is endothermic in both the cases
D
all the steps are exothermic in both the cases
Answer :
one of the steps is endothermic in both the cases