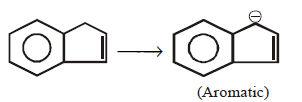
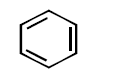
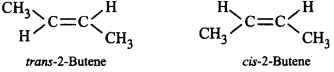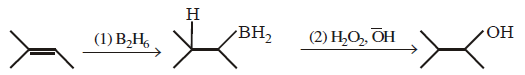41. All the hydrocarbons shown are very weak acids. One, however, is far more acidic than the others. Which one is the strongest acid ?
A

B
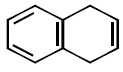

C
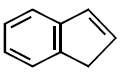

D
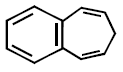

Answer :


42. Baeyer’s reagent is :
A
alkaline permanganate solution
B
acidified permanganate solution
C
neutral permanganate solution
D
aqueous bromine solution
Answer :
alkaline permanganate solution
43. Presence of unsaturation in organic compounds can be tested with
A
Fehling's reagent
B
Tollens' reagent
C
Baeyer's reagent
D
Fittig's reaction
Answer :
Baeyer's reagent
44. The treatment of $$C{H_3}MgX$$ with $$C{H_3}C \equiv C - H$$ produces
A
$$C{H_3} - CH = C{H_2}$$
B
$$C{H_3}C \equiv C - C{H_3}$$
C
\[C{{H}_{3}}-\overset{\begin{smallmatrix}
H \\
|
\end{smallmatrix}}{\mathop{C}}\,=\overset{\begin{smallmatrix}
H \\
|
\end{smallmatrix}}{\mathop{C}}\,-C{{H}_{3}}\]
D
$$C{H_4}$$
Answer :
$$C{H_4}$$
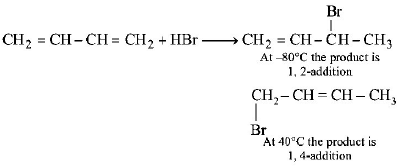
45. Reaction of one molecule of $$HBr$$ with one molecule of 1, 3 - butadiene at $${40^ \circ }C$$ gives predominantly
A
1 - bromo - 2 - butene under kinetically controlled
conditions
B
3 - bromobutene under thermodynamically controlled predominantly
conditions
C
1 - bromo - 2 - butene under thermodynamically controlled conditions
D
3 - bromobutene under kinetically controlled conditions
Answer :
1 - bromo - 2 - butene under thermodynamically controlled conditions
46. How many structures are possible for $${C_5}{H_8}$$ with one triple bond?
A
4
B
3
C
2
D
1
Answer :
3
47. The most acidic hydrogen atoms are present in
A
ethane
B
ethene
C
ethyne
D
benzene
Answer :
ethyne
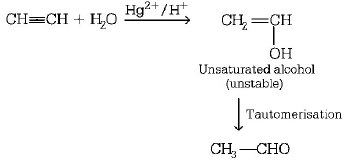
48. When acetylene is passed through $$dil \cdot {H_2}S{O_4}$$ in presence of $$HgS{O_4},$$ the compound formed is
A
ether
B
ketone
C
acetic acid
D
acetaldehyde
Answer :
acetaldehyde
49. The alkene that exhibits geometrical isomerism is
A
propene
B
2-methylpropene
C
2-butene
D
2-methyl-2-butene
Answer :
2-butene
50.
The major product of the following reaction sequence is :
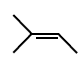 \[\xrightarrow[2.\,{{H}_{2}}{{O}_{2}},\,H{{O}^{-}}]{1.\,{{B}_{2}}{{H}_{6}}}?\]
\[\xrightarrow[2.\,{{H}_{2}}{{O}_{2}},\,H{{O}^{-}}]{1.\,{{B}_{2}}{{H}_{6}}}?\]
A
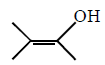

B
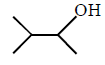

C
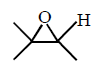

D
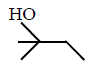

Answer :