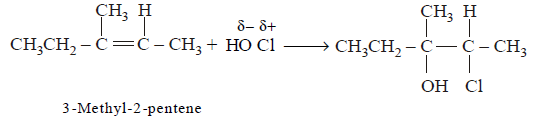51. Which of the following alkynes can be identified and distinguished from the rest of the alkynes on reaction with ammoniacal silver nitrate?
A
$$C{H_3}C \equiv C - C{H_3}$$
B
$$C{H_3}C{H_2}C \equiv CH$$
C
$$C{H_3}C{H_2}C \equiv CC{H_3}$$
D
$$C{H_3}C \equiv CC{H_2}C{H_2}C{H_3}$$
Answer :
$$C{H_3}C{H_2}C \equiv CH$$
52.
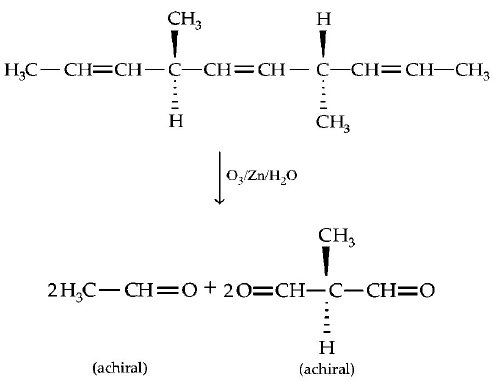
The number of optically active products obtained from the
complete ozonolysis of the given compound is :
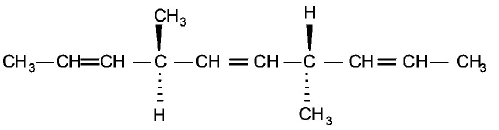
A
0
B
1
C
2
D
4
Answer :
0
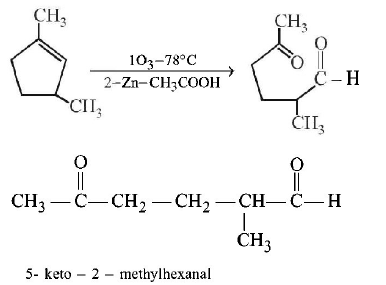
53. Which compound would give 5 - keto - 2 - methylhexanal ipon ozonolysis ?
A
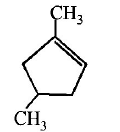

B
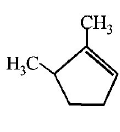

C
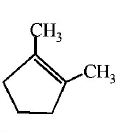

D
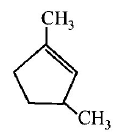

Answer :


54. A compound $$\left( X \right)\left( {{C_5}{H_8}} \right)$$ reacts with ammonical $$AgN{O_3}$$ to give a white precipitate, and on oxidation with hot alkaline $$KMn{O_4}$$ gives the acid, $${\left( {C{H_3}} \right)_2}CHCOOH,$$ therefore $$X$$ is –
A
$$C{H_2} = CH - CH = CH - C{H_3}$$
B
$$C{H_3} - CH = CH - C{H_2} - C{H_3}$$
C
$${\left( {C{H_3}} \right)_2}CH - C \equiv CH$$
D
$${\left( {C{H_3}} \right)_2}C = C = C{H_2}$$
Answer :
$${\left( {C{H_3}} \right)_2}CH - C \equiv CH$$
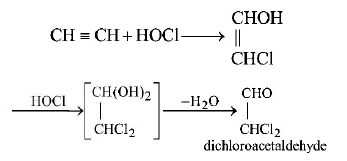
55. What is the product when acetylene reacts with the formation of hypochlorous acid ?
A
$$C{H_3}COCl$$
B
$$ClC{H_2}CHO$$
C
$$C{l_2}CHCHO$$
D
$$ClCHCOOH$$
Answer :
$$C{l_2}CHCHO$$
56. The product$$(s)$$ obtained via oxymercuration $$\left( {HgS{O_4} + {H_2}S{O_4}} \right)$$ of 1- butyne would be
A
\[C{{H}_{3}}-C{{H}_{2}}\overset{\begin{smallmatrix}
O \\
\parallel
\end{smallmatrix}}{\mathop{-C-}}\,C{{H}_{3}}\]
B
$$C{H_3} - C{H_2} - C{H_2} - CHO$$
C
$$C{H_3} - C{H_2} - CHO + HCHO$$
D
$$C{H_3}C{H_2}COOH + HCOOH$$
Answer :
\[C{{H}_{3}}-C{{H}_{2}}\overset{\begin{smallmatrix}
O \\
\parallel
\end{smallmatrix}}{\mathop{-C-}}\,C{{H}_{3}}\]
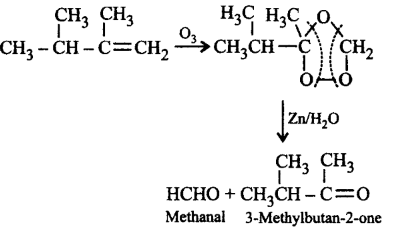
57. Ozonolysis of 2, 3-dimethylbut-1-ene followed by reduction with zinc and water gives
A
methanal and hexanoic acid
B
methanoic acid and butanone
C
methanal and 3-methylbutan-2-one
D
butanoic acid and 2, 3-dimethylbutanoic acid
Answer :
methanal and 3-methylbutan-2-one
58.
Arrange the following in decreasing order of their boiling points
(i) $$n$$ - Butane
(ii) 2 - Methylbutane
(iii) $$n$$ - Pentane
(iv) 2, 2 - Dimethylpropane
A
(i) > (ii) > (iii) > (iv)
B
(ii) > (iii) > (iv) > (i)
C
(iv) > (iii) > (ii) > (i)
D
(iii) > (ii) > (iv) > (i)
Answer :
(iii) > (ii) > (iv) > (i)
59. $$HOCl$$ reacts on 3-methyl-2-pentene, the main product will be :
A


B
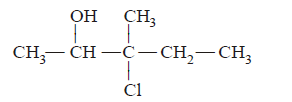
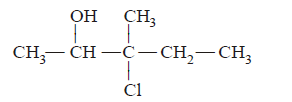
C


D


Answer :


60. Benzene easily shows
A
ring fission reactions since it is unstable
B
addition reactions since it is unsaturated
C
electrophilic substitution reactions due to stable ring and high $$\pi $$ electron density
D
nucleophilic substitution reactions due to stable ring and minimum electron density.
Answer :
electrophilic substitution reactions due to stable ring and high $$\pi $$ electron density