1. Cyclohexylamine is stronger base than aniline because
A
in aniline electron pair is involved in conjugation
B
in cyclohexylamine electron pair is involved in conjugation
C
in aniline $$ - N{H_2}$$ group is protonated
D
in cyclohexylamine nitrogen has a negative charge
Answer :
in aniline electron pair is involved in conjugation
2. The order of basicity of amines in gaseous state is :
A
\[{{1}^{\circ }}>{{2}^{\circ }}>{{3}^{\circ }}>N{{H}_{3}}\]
B
\[{{3}^{\circ }}>{{2}^{\circ }}>N{{H}_{3}}>{{1}^{\circ }}\]
C
\[{{3}^{\circ }}>{{2}^{\circ }}>{{1}^{\circ }}>N{{H}_{3}}\]
D
\[N{{H}_{3}}>{{1}^{\circ }}>{{2}^{\circ }}>{{3}^{\circ }}\]
Answer :
\[{{3}^{\circ }}>{{2}^{\circ }}>{{1}^{\circ }}>N{{H}_{3}}\]
3. Indicate which nitrogen compound amongst the following would undergo Hofmann reaction?
A
$$RCONHC{H_3}$$
B
$$RCOON{H_4}$$
C
$$RCON{H_2}$$
D
$$RCONHOH$$
Answer :
$$RCON{H_2}$$
4.
Coupling of diazonium salts of following takes place in the order
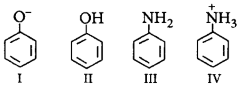
A
IV < II < III < I
B
IV > III < II < I
C
II < IV < I < III
D
I < II < III < IV
Answer :
IV < II < III < I
5. In the Hofmann bromamide degradation reaction, the number of moles of $$NaOH$$ and $$B{r_2}$$ used per mole of amine produced are:
A
Two moles of $$NaOH$$ and two moles of $$B{r_2}$$
B
Four moles of $$NaOH$$ and one mole of $$B{r_2}$$
C
One mole of $$NaOH$$ and one mole of $$B{r_2}$$
D
Four moles of $$NaOH$$ and two moles of $$B{r_2}$$
Answer :
Four moles of $$NaOH$$ and one mole of $$B{r_2}$$
6.
 $$ + C{H_3}MgBr \to P$$
$$ + C{H_3}MgBr \to P$$
Product $$P$$ in the above reaction is
A

B


C


D


Answer :


7.
In the chemical reactions,
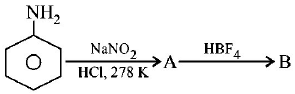
the compounds $$'A'$$ and $$'B'$$ respectively are
A
nitrobenzene and fluorobenzene
B
phenol and benzene
C
benzene diazonium chloride and fluorobenzene
D
nitrobenzene and chlorobenzene
Answer :
benzene diazonium chloride and fluorobenzene
8. Electrolytic reduction of nitrobenzene in weakly acidic medium gives
A
aniline
B
nitrosobenzene
C
$$N$$ - phenyl hydroxylamine
D
$$p$$ - hydroxyaniline
Answer :
aniline
9. Which of the following does not give $$N–$$ ethyl cyclopentylamine as major product ?
A


B
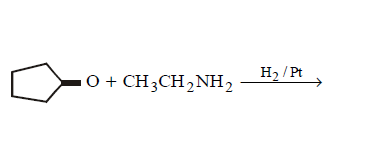
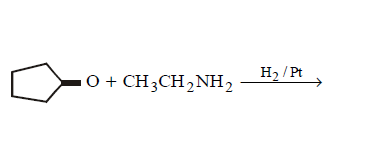
C


D


Answer :


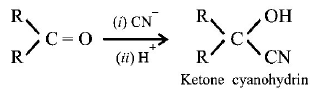
10. The formation of cyanohydrin from a ketone is an example of :
A
Electrophilic addition
B
Nucleophilic addition
C
Nucleophilic substitution
D
Electrophilic substiution
Answer :
Nucleophilic addition