121. Which one of the following pairs is not correctly matched?
A
Terylene-condensation polymer of terephthalic acid and ethylene glycol
B
Teflon-thermally stable cross-linked polymer of phenol and formaldehyde
C
Perspex-homopolymer of methylmethacrylate
D
Synthetic rubber-a copolymer of butadiene and styrene
Answer :
Teflon-thermally stable cross-linked polymer of phenol and formaldehyde
122. Heating rubber with sulphur is known as
A
galvanisation
B
bessemerisation
C
vulcanisation
D
sulphonation
Answer :
vulcanisation
123. Which of the following structures represents neoprene polymer?
A


B


C


D


Answer :


124.
Structures of some common polymers are given. Which one is not correctly presented?
A


B


C


D


Answer :


125. Which one of the following structures represents nylon 6, 6 polymer?
A


B
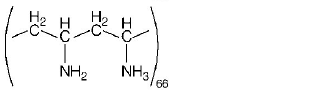

C


D


Answer :


126. Natural rubber has
A
All $$trans$$ - configuration
B
Alternate $$cis$$ - and $$trans$$ - configuration
C
Random $$cis$$ - and $$trans$$ - configuration
D
All $$cis$$ - configuration
Answer :
All $$cis$$ - configuration
127. Among the following, the wrong statement is
A
$$PMMA$$ is plexiglass
B
$$SBR$$ is natural rubber
C
$$PTFE$$ is teflon
D
$$LDPE$$ is low density polythene
Answer :
$$SBR$$ is natural rubber
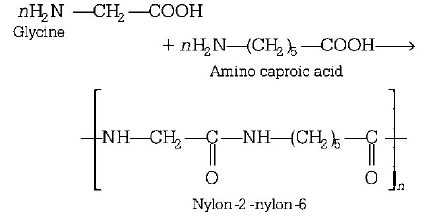
128. Biodegradable polymer which can be produced from glycine and aminocapric acid is
A
nylon 2-nylon 6
B
$$PHBV$$
C
buna - $$N$$
D
nylon - 6, 6
Answer :
nylon 2-nylon 6
129. Polymer used in bullet proof glass is
A
lexan
B
$$PMMA$$
C
nomex
D
kevlar
Answer :
$$PMMA$$
130.
The correct functional group $$X$$ and the reagent / reaction conditions $$Y$$ in the following scheme are
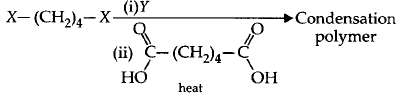
$$\eqalign{
& \left( {\text{i}} \right)X = COOC{H_3},Y = {H_2}/Ni/{\text{heat}} \cr
& \left( {{\text{ii}}} \right)X = CON{H_2},Y = {H_2}/Ni/{\text{heat}} \cr
& \left( {{\text{iii}}} \right)X = CON{H_2},Y = B{r_2}/NaOH \cr
& \left( {{\text{iv}}} \right)X = CN,Y = {H_2}/Ni/{\text{heat}} \cr} $$
A
(i) and (ii)
B
(i), (ii) and (iii)
C
(i) and (iii)
D
All of these
Answer :
All of these