1. If uncertainty principle is applied to an object of mass 1 milligram, the uncertainty value of velocity and position will be
A
$$0.2 \times {10^{ - 4}}\,{m^2}\,{s^{ - 1}}$$
B
$$0.52 \times {10^6}\,{m^2}\,{s^{ - 1}}$$
C
$$0.52 \times {10^{ - 28}}\,{m^2}\,{s^{ - 1}}$$
D
$$2 \times {10^{ - 34}}\,{m^2}\,{s^{ - 1}}$$
Answer :
$$0.52 \times {10^{ - 28}}\,{m^2}\,{s^{ - 1}}$$
2. Any $$p-$$orbital can accommodate upto
A
four electrons
B
six electrons
C
two electrons with parallel spins
D
two electrons with opposite spins
Answer :
two electrons with opposite spins
3. The $$L{i^{2 + }}\,ion$$ is moving in the third stationary state, and its linear momentum is $$7.3 \times {10^{ - 34}}kgm{s^{ - 1}}.$$ Angular momentum is.
A
$$1.158 \times {10^{ - 45}}kg\,{m^2}{s^{ - 1}}$$
B
$$11.58 \times {10^{ - 48}}kg\,{m^2}{s^{ - 1}}$$
C
$$11.58 \times {10^{ - 47}}kg\,{m^2}{s^{ - 1}}$$
D
$$12 \times {10^{ - 45}}kg\,{m^2}{s^{ - 1}}$$
Answer :
$$11.58 \times {10^{ - 48}}kg\,{m^2}{s^{ - 1}}$$
4. If the nitrogen atom has electronic configuration $$1{s^7},$$ it would have energy lower than that of the normal ground state configuration $$1{s^2}2{s^2}2{p^3},$$ because the electrons would be closer to the nucleus. Yet $$1{s^7}$$ is not observed because it violates.
A
Heisenberg uncertainty principle
B
Hund's rule
C
Pauli exclusion principle
D
Bohr postulate of stationary orbits
Answer :
Pauli exclusion principle
5.
Which of the following types of spectrum is best depicted by the given figure ?
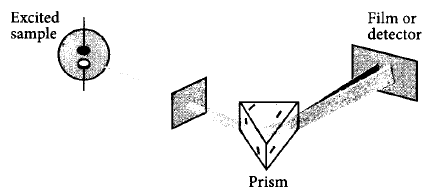
A
Atomic absorption spectra
B
Atomic emission spectra
C
Continuous spectra
D
None of these
Answer :
Atomic emission spectra
6. The position of both, an electron and a helium atom is known within $$1.0\,nm.$$ Further the momentum of the electron is known within $$5.0 \times {10^{ - 26}}kg\,m{s^{ - 1}}.$$ The minimum uncertainty in the measurement of the momentum of the helium atom is
A
$$50\,kg\,m{s^{ - 1}}$$
B
$$80\,kg\,m{s^{ - 1}}$$
C
$$8.0 \times {10^{ - 26}}kg\,m{s^{ - 1}}$$
D
$$5.0 \times {10^{ - 26}}kg\,m{s^{ - 1}}$$
Answer :
$$5.0 \times {10^{ - 26}}kg\,m{s^{ - 1}}$$
7. An electron can enter into the orbital when
A
value of $$n$$ is minimum
B
value of $$l$$ is minimum
C
value of $$\left( {n + l} \right)$$ is minimum
D
value of $$\left( {n + m} \right)$$ is minimum.
Answer :
value of $$\left( {n + l} \right)$$ is minimum
8. Which of the following is not a correct statement regarding the energies of orbitals ?
A
The lower the value of $$\left( {n + l} \right)$$ for an orbital, lower is its energy.
B
Electrons in the same subshell have equal energy.
C
Energy of $$s$$ - orbital is lower than the $$p$$ - orbital and that of $$p$$ - orbital is lower than the $$d$$ - orbital.
D
If two orbitals have same value for $$\left( {n + l} \right),$$ the orbital with higher value of $$n$$ will have lower energy.
Answer :
If two orbitals have same value for $$\left( {n + l} \right),$$ the orbital with higher value of $$n$$ will have lower energy.
9. What will be the mass of a particle if uncertainty in its posttton is $${10^{ - 8}}\,m$$ and velocity is $$5.26 \times {10^{ - 25}}\,m\,{s^{ - 1}}\,?$$
A
0.01$$\,kg$$
B
0.1$$\,kg$$
C
1$$\,kg$$
D
10$$\,kg$$
Answer :
0.01$$\,kg$$
10. An electron, $${e_1}$$ is moving in the fifth stationary state, and another electron $${e_2}$$ is moving in the fourth stationary state. The radius of orbit of electron, $${e_1}$$ is five times the radius of orbit of electron, $${e_2}$$ calculate the ratio of velocity of electron $${e_1}\left( {{v_1}} \right)$$ to the velocity of electron $${e_2}\left( {{v_2}} \right).$$
A
5 : 1
B
4 : 1
C
1 : 5
D
1 : 4
Answer :
1 : 4