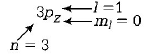11. Who modified Bohr’s theory by introducing elliptical orbits for electron path?
A
Hund
B
Thomson
C
Rutherford
D
Sommerfeld
Answer :
Sommerfeld
12. The number of $$d$$ - electrons in $$F{e^{2 + }}\left( {Z = 26} \right)$$ is not equal to the number of electrons in which one of the following?
A
$$s{\text{ - electrons in }}Mg\left( {Z = 12} \right)$$
B
$$p{\text{ - electrons in }}Cl\left( {Z = 17} \right)$$
C
$$d{\text{ - electrons in }}Fe\,(Z = 26)$$
D
$$p{\text{ - electrons in }}Ne\,(Z = 10)$$
Answer :
$$p{\text{ - electrons in }}Cl\left( {Z = 17} \right)$$
13.
What is the maximum number of orbitals that can be identified with the following quantum numbers?
$$n = 3,\,l = 1,\,$$ and $${m_l} = 0$$
A
1
B
2
C
3
D
4
Answer :
1
14. If the subsidiary quantum number of a subenergy level is 4, the maximum and minimum values of the spin multiplicities are :
A
9, 1
B
10, 1
C
10, 2
D
4, -4
Answer :
10, 2
15. The electron in Bohr's model of hydrogen atom is pictured as revolving around the nucleus in order for it to
A
emit protons
B
keep from being pulled into the nucleus
C
keep from being repelled by the nucleus
D
possess energy.
Answer :
possess energy.
16. The angular momentum of electrons in $$d$$ orbital is equal to
A
$$\sqrt 6 h$$
B
$$\sqrt 2 h$$
C
$$2\sqrt 3 h$$
D
$$0\,h$$
Answer :
$$\sqrt 6 h$$
17. If a proton and $$\alpha $$-particle are accelerated through the same potential difference, the ratio of de-Broglie wavelengths $${\lambda _p}$$ and $${\lambda _\alpha }$$ is
A
$$3$$
B
$$2\sqrt 2 $$
C
$$1$$
D
$$2$$
Answer :
$$2\sqrt 2 $$
18. Which electronic level would allow the hydrogen atom to absorb a photon but not to emit a photon?
A
$$3s$$
B
$$2p$$
C
$$2s$$
D
$$1s$$
Answer :
$$1s$$
19. Two atoms are said to be isobars if
A
they have same atomic number but different mass number
B
they have same number of electrons but different number of neutrons
C
they have same number of neutrons but different number of electrons
D
sum of the number of protons and neutrons is same but the number of protons is different.
Answer :
sum of the number of protons and neutrons is same but the number of protons is different.
20.
Study the orbital diagrams of two atoms $$'X'$$ and $$'Y'.$$ Which subshell will be more stable and why ?

A
$$X,$$ exchange energy is maximum, so is stability
B
$$Y,$$ exchange energy is maximum, so is stability.
C
$$X,$$ exchange energy is minimum, so stability is maximum.
D
$$Y,$$ exchange energy is minimum, so stability is maximum.
Answer :
$$X,$$ exchange energy is maximum, so is stability