21. Correct set of four quantum numbers for the valence (outermost) electron of rubidium $$\left( {Z = 37} \right)$$ is:
A
$$5,0,0, + \frac{1}{2}$$
B
$$5,1,0 + \frac{1}{2}$$
C
$$5,1,1, + \frac{1}{2}$$
D
$$6,0,0, + \frac{1}{2}$$
Answer :
$$5,0,0, + \frac{1}{2}$$
22. Which is the correct order of increasing energy of the listed orbitals in the atom of titanium?
A
$$3s\,\,4s\,\,3p\,\,3d$$
B
$$4s\,\,3s\,\,3p\,\,3d$$
C
$$3s\,\,3p\,\,3d\,\,4s$$
D
$$3s\,\,3p\,\,4s\,\,3d$$
Answer :
$$3s\,\,3p\,\,3d\,\,4s$$
23.
The orbital diagram in which the Aufbau principle is violated is :
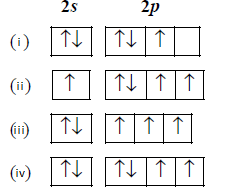
A
(i)
B
(ii)
C
(iii)
D
(iv)
Answer :
(ii)
24. $$P$$ is the probability of finding the $$1s$$ electron of hydrogen atom in a spherical shell of infinitesimal thickness $$dr,$$ at a distance $$r$$ from the nucleus. The volume of this shell is $$4\pi {r^2}dr.$$ The qualitative sketch of the dependence of $$P$$ on $$r$$ is
A


B
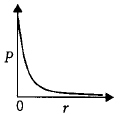

C
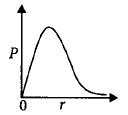

D


Answer :


25. The threshold frequency of a metal is $$1 \times {10^{15}}{s^{ - 1}}.$$ The ratio of the maximum kinetic energies of the photoelectrons when the metal is irradiated with radiations of frequencies $$1.5 \times {10^{15}}{s^{ - 1}}$$ and $$2.0 \times {10^{15}}{s^{ - 1}}$$ respectively would be
A
4 : 3
B
1 : 2
C
2 : 1
D
3 : 4
Answer :
1 : 2
26.
The correct set of quantum numbers for the unpaired electron of chlorine atom is:
| $$n$$ | $$l$$ | $$m$$ | |
| (a) | 2 | 1 | 0 |
| (b) | 2 | 1 | 1 |
| (c) | 3 | 1 | 1 |
| (d) | 3 | 0 | 0 |
A
(a)
B
(b)
C
(c)
D
(d)
Answer :
(c)
27. If $$n =6,$$ the correct sequence for filling of electrons will be
A
$$ns \to \left( {n - 1} \right)d \to \left( {n - 2} \right)f \to np$$
B
$$ns \to \left( {n - 2} \right)f \to np \to \left( {n - 1} \right)d$$
C
$$ns \to np \to \left( {n - 1} \right)d \to \left( {n - 2} \right)f$$
D
$$ns \to \left( {n - 2} \right)f \to \left( {n - 1} \right)d \to np$$
Answer :
$$ns \to \left( {n - 2} \right)f \to \left( {n - 1} \right)d \to np$$
28. If $$m$$ and $$e$$ are the mass and charge of the revolving electron in the orbit of radius $$r$$ for hydrogen atom, the total energy of the revolving electron will be :
A
$$\frac{1}{2}\frac{{{e^2}}}{r}$$
B
$$ - \frac{{{e^2}}}{r}$$
C
$$\frac{{m{e^2}}}{r}$$
D
$$ - \frac{1}{2}\frac{{{e^2}}}{r}$$
Answer :
$$ - \frac{1}{2}\frac{{{e^2}}}{r}$$
29. What will be the wavenumber of yellow radiation having wavelength 240$$\,nm?$$
A
$$1.724 \times {10^4}\,c{m^{ - 1}}$$
B
$$4.16 \times {10^6}\,{m^{ - 1}}$$
C
$$4 \times {10^{14}}\,Hz$$
D
$$219.3 \times {10^3}\,c{m^{ - 1}}$$
Answer :
$$4.16 \times {10^6}\,{m^{ - 1}}$$
30. Rutherford’s experiment on scattering of $$a$$ -particles showed for the first time that the atom has
A
electrons
B
protons
C
nucleus
D
neutrons
Answer :
nucleus