31. The energy of $${e^ - }$$ in first orbit of $$H{e^ + }$$ is $$ - 871.6 \times {10^{ - 20}}J.$$ The energy of $${e^ - }$$ in first orbit of $$H$$ is :
A
$$ - 871.6 \times {10^{ - 20}}$$
B
$$ - 435.8 \times {10^{ - 20}}J$$
C
$$ - 217.9 \times {10^{ - 20}}J$$
D
$$ - 108.9 \times {10^{ - 20}}J$$
Answer :
$$ - 217.9 \times {10^{ - 20}}J$$
32. Which of the following sets of quantum numbers represents the highest energy of an atom?
A
$${\text{n = 3, 1 = 0, m = 0, s = + }}\frac{1}{2}$$
B
$${\text{n = 3, 1 = 1, m = 1, s = + }}\frac{1}{2}$$
C
$${\text{n = 3, 1 = 2, m = 1, s = + }}\frac{1}{2}$$
D
$${\text{n = 4, 1 = 0, m = 0, s = + }}\frac{1}{2}$$
Answer :
$${\text{n = 3, 1 = 2, m = 1, s = + }}\frac{1}{2}$$
33. At temperature $$T,$$ the average kinetic energy of any particle is $$\frac{3}{2}KT.$$ The de Broglie wavelength follows the order :
A
Visible photon > Thermal neutron > Thermal electron
B
Thermal proton > Thermal electron > Visible photon
C
Thermal proton > Visible photon > Thermal electron
D
Visible photon > Thermal electron > Thermal neutron
Answer :
Visible photon > Thermal electron > Thermal neutron
34. In ground state of $$C{u^ + }.$$ The no. of shells occupied, subshells, filled orbitals and unpaired electrons respectively are :
A
4, 8, 15, 0
B
3, 6, 15, 1
C
3, 6, 14, 0
D
4, 7, 14, 2
Answer :
3, 6, 14, 0
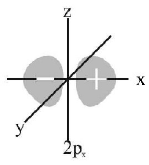
35. The number of nodal planes in $${\text{a}}\,{p_x}$$ orbital is
A
one
B
two
C
three
D
zero
Answer :
one
36. When an electron of charge e and mass $$m$$ moves with a velocity $$v$$ about the nuclear charge $$Ze$$ in circular orbit of radius $$r,$$ the potential energy of the electrons is given by
A
$$\frac{{{Z^2}{e^2}}}{r}$$
B
$$ - \frac{{Z{e^2}}}{r}$$
C
$$\frac{{Z{e^2}}}{r}$$
D
$$\frac{{m{v^2}}}{r}$$
Answer :
$$ - \frac{{Z{e^2}}}{r}$$
37.
What is the maximum numbers of electrons that can be associated with the following set of quantum numbers?
$$n = 3,l = 1\,{\text{and}}\,m = - 1$$
A
10
B
6
C
4
D
2
Answer :
2
38. Two values of spin quantum numbers i.e., $$ + \frac{1}{2}$$ and $$ - \frac{1}{2}$$ represent
A
up and down spin of the electrons respectively
B
two quantum mechanical spin states which refer to the orientation of spin of the electron
C
anti-clockwise and clockwise spin of the electrons respectively.
D
none of these
Answer :
two quantum mechanical spin states which refer to the orientation of spin of the electron
39. The configuration of the valence orbital of an element with atomic number 22 is
A
$$3{d^5}4{s^1}$$
B
$$4{s^2}3{d^2}$$
C
$$4{s^1}4{p^1}$$
D
$$3{d^2}4{s^1}4{p^1}$$
Answer :
$$4{s^2}3{d^2}$$
40. Which of the following statements about Thomson model of atom is correct ?
A
An atom possesses an elliptical shape in which the positive charge is uniformly distributed.
B
Mass of the atom is assumed to be uniformly distributed over the atom.
C
This model was unable to explain the overall neutrality of the atom.
D
All of these
Answer :
Mass of the atom is assumed to be uniformly distributed over the atom.