81. Number of angular nodes for $$4d$$ orbital is ____________.
A
4
B
3
C
2
D
1
Answer :
2
82. The velocity of an electron in a certain Bohr orbit of $$H$$ - atom bears the ratio 1 : 275 to the velocity of light. The quantum number $$(n)$$ of the orbit is
A
3
B
2
C
1
D
4
Answer :
2
83. The wavelength of the radiation emitted, when in a hydrogen atom electron falls from infinity to stationary state 1, would be ( Rydberg constant $$ = 1.097 \times {10^7}{m^{ - 1}}$$ )
A
$$406\,nm$$
B
$$192\,nm$$
C
$$91\,nm$$
D
$$9.1 \times {10^{ - 8}}nm$$
Answer :
$$91\,nm$$
84. Maximum number of electrons in a subshell with $$l = 3$$ and $$n = 4$$ is
A
14
B
16
C
10
D
12
Answer :
14
85.
Which one of the following sets of quantum numbers represents an impossible arrangement?
| n | l | $${m_l}$$ | $${m_s}$$ | |
| (a) | 3 | 2 | −2 | $$\frac{1}{2}$$ |
| (b) | 4 | 0 | 0 | $$\frac{1}{2}$$ |
| (c) | 3 | 2 | −3 | $$\frac{1}{2}$$ |
| (d) | 5 | 3 | 0 | $$ - \frac{1}{2}$$ |
A
(a)
B
(b)
C
(c)
D
(d)
Answer :
(c)
86. If the ionisation energy of hydrogen atom is 13.6$$\,eV,$$ the energy required to excite it from ground state to the next higher state is approximately
A
3.4$$\,eV$$
B
10.2$$\,eV$$
C
17.2$$\,eV$$
D
13.6$$\,eV$$
Answer :
3.4$$\,eV$$
87. If in Bohr's model, for unielectronic atom, time period of revolution is represented as $${T_{n,z}}$$ where $$n$$ represents shell no. and $$Z$$ represents atomic number then the value of $${T_{1,2}}:{T_{2,1}}$$ will be :
A
8 : 1
B
1 : 8
C
1 : 1
D
1 : 32
Answer :
1 : 32
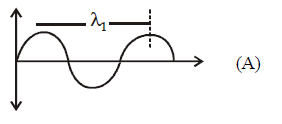
88.
What will be the difference between electromagnetic radiation shown in $$A$$ and $$B$$ respectively ?

(i) Velocity
(ii) Wavelength
(iii) Frequency
(iv) Energy
A
(ii) only
B
(ii) and (iv)
C
(ii), (iii) and (iv)
D
(iv) only
Answer :
(ii), (iii) and (iv)
89. Which of the following options does not represent ground state electronic configuration of an atom ?
A
$$1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^8}4{s^2}$$
B
$$1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^9}4{s^2}$$
C
$$1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^{10}}4{s^1}$$
D
$$1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^5}4{s^1}$$
Answer :
$$1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^9}4{s^2}$$
90. Which of the following statements is false ?
A
Photon has momentum as well as wavelength.
B
Splitting of spectral lines in electrical field is called Stark effect.
C
Frequency of emitted radiation from a black body goes from a lower wavelength to higher wavelength as the temperature increases.
D
Rydberg constant has unit of energy.
Answer :
Frequency of emitted radiation from a black body goes from a lower wavelength to higher wavelength as the temperature increases.