131. The entropy change in the fusion of one mole of a solid melting at $${27^ \circ }C$$ ( latent heat of fusion is $$2930\,J\,mo{l^{ - 1}}$$ ) is
A
$$9.77\,J\,{K^{ - 1}}\,mo{l^{ - 1}}$$
B
$$10.73\,J{K^{ - 1}}\,mo{l^{ - 1}}$$
C
$$2930\,J\,{K^{ - 1}}\,mo{l^{ - 1}}$$
D
$$108.5\,J\,{K^{ - 1}}\,mo{l^{ - 1}}$$
Answer :
$$9.77\,J\,{K^{ - 1}}\,mo{l^{ - 1}}$$
132. Assuming that water vapour is an ideal gas, the internal energy change $$\left( {\Delta U} \right)$$ when 1 mol of water is vapourised at 1 bar pressure and 100°C, ( given : molar enthalpy of vapourisation of water at 1 bar and $$373\,K = 41\,kJ\,mo{l^{ - 1}}$$ and $$R = 8.3\,J\,mo{l^{ - 1}}{K^{ - 1}}$$ ) will be
A
$$41.00\,kJ\,mo{l^{ - 1}}$$
B
$$4.100\,kJ\,mo{l^{ - 1}}$$
C
$$3.7904\,kJ\,mo{l^{ - 1}}$$
D
$$37.904\,kJ\,mo{l^{ - 1}}$$
Answer :
$$37.904\,kJ\,mo{l^{ - 1}}$$
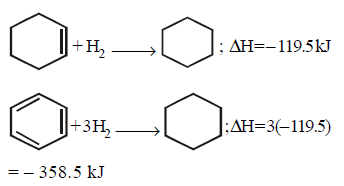
133. The enthalpy of hydrogenation of cyclohexene is $$ - 119.5\,kJ\,mo{l^{ - 1}}.$$ If resonance energy of benzene is $$ - 150.4\,kJ\,mo{l^{ - 1}},$$ its enthalpy of hydrogenation would be
A
$$ - 208.1\,kg\,mo{l^{ - 1}}$$
B
$$ - 269.9\,kg\,mo{l^{ - 1}}$$
C
$$ - 358.5\,kg\,mo{l^{ - 1}}$$
D
$$ - 508.9\,kg\,mo{l^{ - 1}}$$
Answer :
$$ - 208.1\,kg\,mo{l^{ - 1}}$$
134. The enthalpy of solution of sodium chloride is $$4\,kJ\,mo{l^{ - 1}}$$ and its enthalpy of hydration of ions is $$ - 784\,kJ\,mo{l^{ - 1}}.$$ What will be the lattice enthalpy of sodium chloride ?
A
$$ + 780\,kJ\,mo{l^{ - 1}}$$
B
$$ + 394\,kJ\,mo{l^{ - 1}}$$
C
$$ + 788\,kJ\,mo{l^{ - 1}}$$
D
$$ + 398\,kJ\,mo{l^{ - 1}}$$
Answer :
$$ + 788\,kJ\,mo{l^{ - 1}}$$
135. Reaction of methanol with dioxygen was carried out and $$\Delta U$$ was found to be $$ - 726\,kJ\,mo{l^{ - 1}}$$ at $$298\,K.$$ The enthalpy change for the reaction will be $$C{H_3}O{H_{\left( l \right)}} + \frac{3}{2}{O_{2\left( g \right)}} \to C{O_{2\left( g \right)}} + 2{H_2}{O_{\left( l \right)}};$$ $$\Delta H = - 726\,kJ\,mo{l^{ - 1}}$$
A
$$ - 741.5\,kJ\,mo{l^{ - 1}}$$
B
$$ - 727.2\,kJ\,mo{l^{ - 1}}$$
C
$$ + 741.5\,kJ\,mo{l^{ - 1}}$$
D
$$ + 727.2\,kJ\,mo{l^{ - 1}}$$
Answer :
$$ - 727.2\,kJ\,mo{l^{ - 1}}$$
136.
For a reaction, $$CaC{O_{3\left( s \right)}} \to Ca{O_{\left( s \right)}} + C{O_{2\left( g \right)}}$$
$${\Delta _f}{H^ \circ }\left( {CaO} \right) = - 635.1\,kJ\,mo{l^{ - 1}},$$
$${\Delta _f}{H^ \circ }\left( {C{O_2}} \right) = - 393.5\,kJ\,mo{l^{ - 1}}$$ and
$${\Delta _f}{H^ \circ }\left( {CaC{O_3}} \right) = - 1206.9\,kJ\,mo{l^{ - 1}}$$
Which of the following is a correct statement ?
A
A large amount of heat is evolved during the decomposition of $$CaC{O_3}.$$
B
Decomposition of $$CaC{O_3}$$ is an endothermic process and heat is provided for decomposition
C
The amount of heat evolved cannot be calculated from the data provided.
D
$${\Delta _r}{H^ \circ } = \sum {{\Delta _f}{H^ \circ }{\text{(reactants)}} - \sum {{\Delta _f}{H^ \circ }{\text{(products)}}} } $$
Answer :
Decomposition of $$CaC{O_3}$$ is an endothermic process and heat is provided for decomposition
137. What is the equilibrium constant if $$ATP$$ hydrolysis by water produce standard free energy of $$ - 50\,kJ/mol$$ under normal body conditions ?
A
$$2.66 \times {10^8}$$
B
$$5.81 \times {10^8}$$
C
$$1.18 \times {10^7}$$
D
$$1.98 \times {10^8}$$
Answer :
$$2.66 \times {10^8}$$
138. $$0.5\,mole$$ each of two ideal gases $$A\left( {{C_{v,m}} = \frac{5}{2}R} \right)$$ and $$B\left( {{C_{v,m}} = 3R} \right)$$ are taken in a container and expanded reversibly and adiabatically, during this process temperature of gaseous mixture decreased from $$350\,K$$ to $$250\,K.$$ Find $$\Delta H$$ ( in $$cal/mol$$ ) for the process :
A
$$ - 100\,R$$
B
$$ - 137.5\,R$$
C
$$ - 375\,R$$
D
$${\text{None of these}}$$
Answer :
$$ - 375\,R$$
139.
Combustion of sucrose is used by aerobic organisms for providing energy for the life
sustaining processes. If all the capturing of energy from the reaction is done through
electrical process ( non $$P–V$$ work ) then calculate maximum available energy which can be captured by combustion of $$34.2\,g$$ of sucrose
Given : $$\Delta {H_{{\text{combustion}}}}{\text{(sucrose)}} = - 6000\,kJ\,mo{l^{ - 1}}$$
$$\Delta {S_{{\text{combustion}}}} = 180\,J/K\,mol$$ and body temperature is $$300\,K$$
A
600$$\,kJ$$
B
594.6$$\,kJ$$
C
5.4$$\,kJ$$
D
605.4$$\,kJ$$
Answer :
605.4$$\,kJ$$
140. For the reaction $${C_3}{H_8}\left( g \right) + 5{O_2}\left( g \right) \to $$ $$3C{O_2}\left( g \right) + 4{H_2}O\left( l \right)$$ at constant temperature, $$\Delta H - \Delta E$$ is
A
$$ - RT$$
B
$$ + RT$$
C
$$ - 3\,RT$$
D
$$ + 3\,RT$$
Answer :
$$ - 3\,RT$$