201. The conjugate acid of $$NH_2^ - $$ is :
A
$$N{H_3}$$
B
$$N{H_2}OH$$
C
$$NH_4^ + $$
D
$${N_2}{H_4}$$
Answer :
$$N{H_3}$$
202. What would be the $$pH$$ of a solution obtained by mixing $$5g$$ of acetic acid and $$7.5g$$ of sodium acetate and making the volume equal to $$500\,mL?$$ $$\left( {{K_a} = 1.75 \times {{10}^{ - 5}},p{K_a} = 4.76} \right)$$
A
$$pH = 4.70$$
B
$$pH < 4.70$$
C
$$pH$$ of solution will be equal to $$pH$$ of acetic acid
D
$$4.76 < pH < 5.0$$
Answer :
$$4.76 < pH < 5.0$$
203. Which of the following salts with a concentration $$1.0\,M$$ will give a basic solution?
A
Ammonium acetate
B
Ammonium chloride
C
Ammonium sulphate
D
Sodium acetate
Answer :
Sodium acetate
204.
Values of dissociation constant, $${K_a}$$ are given as follows :
$$\eqalign{
& {\text{Acid}}\,\,\,\,\,\,\,{K_a} \cr
& HCN\,\,\,\,\,6.2 \times {10^{ - 10}} \cr
& HF\,\,\,\,\,\,\,\,\,\,7.2 \times {10^{ - 4}} \cr
& HN{O_2}\,\,\,4.0 \times {10^{ - 4}} \cr} $$
Correct order of increasing base strength of the base $$C{N^ - },{F^ - }$$ and $$NO_2^ - $$ will be :
A
$${F^ - } < C{H^ - } < NO_2^ - $$
B
$$NO_2^ - < C{N^ - } < {F^ - }$$
C
$${F^ - } < NO_2^ - < C{N^ - }$$
D
$$NO_2^ - < {F^ - } < C{N^ - }$$
Answer :
$${F^ - } < NO_2^ - < C{N^ - }$$
205. The solubility of $$AgCl\left( s \right)$$ with solubility product $$1.6 \times {10^{ - 10}}$$ in $$0.1\,M\,NaCl$$ solution would be
A
$$1.26 \times {10^{ - 5}}M$$
B
$$1.6 \times {10^{ - 9}}M$$
C
$$1.6 \times {10^{ - 11}}M$$
D
$${\text{zero}}$$
Answer :
$$1.6 \times {10^{ - 9}}M$$
206. The $$pH$$ of a 0.1 molar solution of the acid $$HQ$$ is 3. The value of theionizalion constant, $${K_a}$$ the acid is :
A
$$3 \times {10^{ - 1}}$$
B
$$1 \times {10^{ - 3}}$$
C
$$1 \times {10^{ - 5}}$$
D
$$1 \times {10^{ - 7}}$$
Answer :
$$1 \times {10^{ - 5}}$$
207. $$N{H_4}CN$$ is a salt of weak acid $$HCN\left( {{K_a} = 6.2 \times {{10}^{ - 10}}} \right)$$ and a weak base $$N{H_4}OH\left( {{K_b} = 1.8 \times {{10}^{ - 5}}} \right).$$ A one molar solution of $$N{H_4}CN$$ will be
A
neutral
B
strongly acidic
C
strongly basic
D
weakly basic
Answer :
weakly basic
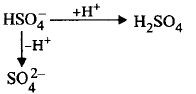
208. Which of the following species can act both as an acid as well as base?
A
$$SO_4^{2 - }$$
B
$$HSO_4^ - $$
C
$$PO_4^{3 - }$$
D
$$C{l^ - }$$
Answer :
$$HSO_4^ - $$
209.
The following equilibrium is established when hydrogen chloride is dissolved in acetic acid.
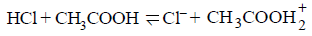
The set that characterises the conjugate acidbase pairs is
A
$$\left( {HCl,C{H_3}COOH} \right){\text{and}}\left( {C{H_3}COOH_2^ + ,C{l^ - }} \right)$$
B
$$\left( {HCl,C{H_3}COOH_2^ + } \right){\text{and}}\left( {C{H_3}COOH,C{l^ - }} \right)$$
C
$$\left( {C{H_3}COOH_2^ + ,HCl} \right){\text{and}}\left( {C{l^ - },C{H_3}COOH} \right)$$
D
$$\left( {HCl,C{l^ - }} \right){\text{and}}\left( {C{H_3}COOH_2^ + ,C{H_3}COOH} \right)$$
Answer :
$$\left( {HCl,C{l^ - }} \right){\text{and}}\left( {C{H_3}COOH_2^ + ,C{H_3}COOH} \right)$$
210. The compound whose $$0.1 M$$ solution is basic is :
A
ammonium acetate
B
ammonium chloride
C
ammonium sulphate
D
sodium acetate
Answer :
sodium acetate