91.
Which of the following is true about the given redox reaction?
$$SnC{l_2} + 2FeC{l_3} \to SnC{l_4} + 2FeC{l_2}$$
A
$$SnC{l_2}$$ is oxidised and $$FeC{l_3}$$ acts as oxidising agent.
B
$$FeC{l_3}$$ is oxidised and acts as oxidising agent.
C
$$SnC{l_2}$$ is reduced and acts as oxidising agent.
D
$$FeC{l_3}$$ is oxidised and $$SnC{l_2}$$ acts as oxidising agent.
Answer :
$$SnC{l_2}$$ is oxidised and $$FeC{l_3}$$ acts as oxidising agent.
92. When $$S{O_2}$$ is passed through acidified solution of potassium dichromate, then chromium sulphate is formed. The change in valency of chromium is
A
+4 to +2
B
+5 to +3
C
+6 to +3
D
+7 to +2
Answer :
+6 to +3
93. The brown ring complex is formulated as $$\left[ {Fe{{\left( {{H_2}O} \right)}_5}NO} \right]S{O_4}.$$ The oxidation number of iron is
A
1
B
2
C
3
D
0
Answer :
1
94. $$MnO_4^ - $$ ions are reduced in acidic condition to $$M{n^{2 + }}$$ ions whereas they are reduced in neutral condition to $$Mn{O_2}.$$ The oxidation of $$25\,mL$$ of a solution $$X$$ containing $$F{e^{2 + }}$$ ions required in acidic condition $$20\,mL$$ of a solution $$Y$$ containing $$MnO_4^ - $$ ions. What volume of solution $$Y$$ would be required to oxidise $$25\,mL$$ of solution $$X$$ containing $$F{e^{2 + }}$$ ions in neutral condition?
A
11.4$$\,mL$$
B
12.0 $$mL$$
C
33.3$$\,mL$$
D
35.0$$\,mL$$
Answer :
33.3$$\,mL$$
95. Which of the following oxidation numbers is not correctly matched?
A
$$P\,\,{\text{in}}\,\,Na{H_2}P{O_4} = + 5$$
B
$$Ni\,\,{\text{in}}\,\,{\left[ {Ni{{\left( {CN} \right)}_6}} \right]^{4 - }} = + 2$$
C
$$P\,\,{\text{in}}\,\,M{g_2}{P_2}{O_7} = + 6$$
D
$$Cr\,\,{\text{in}}\,\,{\left( {N{H_4}} \right)_2}C{r_2}{O_7} = + 6$$
Answer :
$$P\,\,{\text{in}}\,\,M{g_2}{P_2}{O_7} = + 6$$
96. Examples of few compounds are given in a particular oxidation state. Mark the example which is not correct.
A
Phosphorus in +1 oxidation state $$ - {H_3}P{O_2}$$
B
Chlorine in +7 oxidation state $$ - HClO$$
C
Chromium in +6 oxidation state $$ - Cr{O_2}C{l_2}$$
D
Carbon in 0 oxidation state $$ - {C_{12}}{H_{22}}{O_{11}}$$
Answer :
Chlorine in +7 oxidation state $$ - HClO$$
97. One mole of $${N_2}{H_4}$$ loses $$10\,moles$$ of electrons to form a new compound, $$y.$$ Assuming that all nitrogen appear in the new compound, what is the oxidation state of nitrogen in $$y$$ ( There is no change in the oxidation state of hydrogen )
A
$$-1$$
B
$$-3$$
C
$$+3$$
D
$$+5$$
Answer :
$$+3$$
98.
Which species is acting as a reducing agent in the following reaction?
$$14{H^ + } + C{r_2}O_7^{2 - } + 3Ni \to $$ $$2C{r^{3 + }} + 7{H_2}O + 3N{i^{2 + }}$$
A
$$C{r_2}O_7^{2 - }$$
B
$$Ni$$
C
$${H^ + }$$
D
$${H_2}O$$
Answer :
$$Ni$$
99.
Thiosulphate reacts differently with iodine and bromine in the reactions given below :
$$2{S_2}O_3^{2 - } + {I_2} \to {S_4}O_6^{2 - } + 2{I^ - }$$
$${S_2}O_3^{2 - } + 2B{r_2} + 5{H_2}O \to $$ $$2SO_4^{2 - } + 2B{r^ - } + 10{H^ + }$$
Which of the following statements justifies the above dual behaviour of thiosulphate?
A
Bromine is a stronger oxidant than iodine.
B
Bromine is a weaker oxidant than iodine.
C
Thiosulphate undergoes oxidation by bromine and reduction by iodine in these reactions.
D
Bromine undergoes oxidation and iodine undergoes reduction in these reactions.
Answer :
Bromine is a stronger oxidant than iodine.
100.
A redox reaction is shown in the diagrams. Identify the reaction.
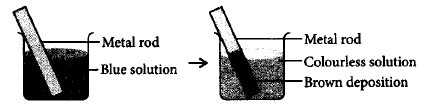
A
$$Z{n_{\left( s \right)}} + Cu_{\left( {aq} \right)}^{2 + } \to Zn_{\left( {aq} \right)}^{2 + } + C{u_{\left( s \right)}}$$
B
$$C{u_{\left( s \right)}} + 2Ag_{\left( {aq} \right)}^ + \to Cu_{\left( {aq} \right)}^{2 + } + 2A{g_{\left( s \right)}}$$
C
$$2A{g_{\left( s \right)}} + Cu_{\left( {aq} \right)}^{2 + } \to 2Ag_{\left( {aq} \right)}^ + + C{u_{\left( s \right)}}$$
D
$$C{u_{\left( s \right)}} + Zn_{\left( {aq} \right)}^{2 + } \to Cu_{\left( {aq} \right)}^{2 + } + Z{n_{\left( s \right)}}$$
Answer :
$$Z{n_{\left( s \right)}} + Cu_{\left( {aq} \right)}^{2 + } \to Zn_{\left( {aq} \right)}^{2 + } + C{u_{\left( s \right)}}$$