161. Select the correct statement. In the gas equation $$pV = nRT$$
A
$$n$$ is the number of molecules of a gas
B
$$V$$ denotes volume of one mole of the gas
C
$$n$$ moles of the gas have a volume $$V$$
D
$$p$$ is the pressure of the gas when only one mole of the gas is present
Answer :
$$n$$ moles of the gas have a volume $$V$$
162. At $${27^ \circ }C$$ a sample of ammonia gas exerts a pressure of 5.3 atm. What is the pressure when the volume of the gas is reduced to one-tenth of the original value at the same temperature?
A
0.53$$\,atm$$
B
5.3$$\,atm$$
C
53$$\,atm$$
D
None of these
Answer :
53$$\,atm$$
163. For orthorhombic system axial ratios are $$a \ne b \ne c$$ and the axial angles are
A
$$\alpha = \beta = \gamma \ne {90^ \circ }$$
B
$$\alpha = \beta = \gamma = {90^ \circ }$$
C
$$\alpha = \gamma = {90^ \circ },\beta \ne {90^ \circ }$$
D
$$\alpha \ne \beta \ne \gamma \ne {90^ \circ }$$
Answer :
$$\alpha = \beta = \gamma = {90^ \circ }$$
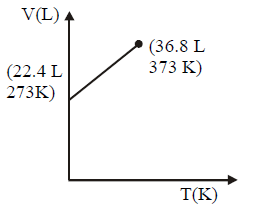
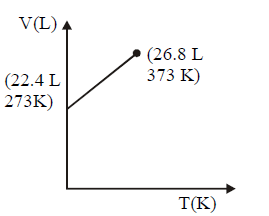
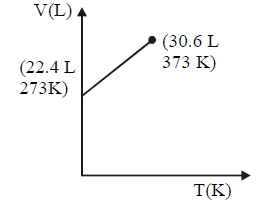
164. Which of the following volume $$(V)$$ - temperature $$(T)$$ plots represents the behaviour of one mole of an ideal gas at one atmospheric pressure ?
A

B

C

D
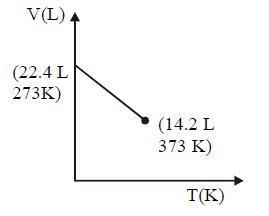

Answer :


165.
Gases possess characteristic critical temperature which depends upon the magnitude of intermolecular forces between the particles. Following are the critical temperatures of some gases.
| Gases | $${H_2}$$ | $$He$$ | $${O_2}$$ | $${N_2}$$ |
| Critical temperature in kelvin | 33.2 | 5.3 | 154.3 | 126 |
From the above data what would be the order of liquefaction of these gases? Start writing the order from the gas liquefying first.
A
$${H_2},He,{O_2},{N_2}$$
B
$$He,{O_2},{H_2},{N_2}$$
C
$${N_2},{O_2},He,{H_2}$$
D
$${O_2},{N_2},{H_2},He$$
Answer :
$${O_2},{N_2},{H_2},He$$
166.
Which of the following statements are correct?
(i) Hydrogen bonding is a special case of dipole - dipole interaction.
(ii) Energy of hydrogen bond varies between $$10$$ to $$100\,kJ\,mo{l^{ - 1}}.$$
(iii) Hydrogen bonds are powerful force in determining the structure and properties of compounds like proteins, nucleic acids etc.
(iv) Strength of the hydrogen bond is determined by the coulombic interaction
between the lone-pair electrons of the electronegative atom of one molecule and
the hydrogen atom of other molecule.
A
(i) and (ii)
B
(i), (ii) and (iii)
C
(ii), (iii) and (iv)
D
All of these
Answer :
All of these
167. An ideal gas, obeying kinetic theory of gases cannot be liquefied, because
A
it solidifies before becoming a liquid
B
forces acting between its molecules are negligible
C
its critical temperature is above $$0{\,^ \circ }C$$
D
its molecules are relatively small in size.
Answer :
forces acting between its molecules are negligible
168. A spherical balloon of $$21\,cm$$ diameter is to be filled with $${H_2}$$ at $$NTP$$ from a cylinder containing the gas at $$20\,atm$$ at $${27^ \circ }C.$$ The cylinder can hold $$2.82\,L$$ of water at $$NTP.$$ The number of balloons that can be filled up is
A
15
B
10
C
20
D
25
Answer :
10
169. The ratio of masses of oxygen and nitrogen in a particular gaseous mixture is 1 : 4. The ratio of number of their molecule is:
A
1 : 4
B
7 : 32
C
1 : 8
D
3 : 16
Answer :
7 : 32
170. The density of neon will be highest at
A
$$S.TP.$$
B
$${0^ \circ }C,\,2\,atm$$
C
$${273^ \circ }C,\,1\,atm$$
D
$${273^ \circ }C,\,2\,atm$$
Answer :
$${0^ \circ }C,\,2\,atm$$