241. A 3 : 2 molar mixture of $${N_2}$$ and $$CO$$ is present in a vessel at $$500\,bar$$ pressure. Due to hole in the vessel, the gas mixture leaks out. The composition of mixture effusing out initially is
A
$${n_{{N_2}}}:{n_{CO}}::1:2$$
B
$${n_{{N_2}}}:{n_{CO}}::6:1$$
C
$${n_{CO}}:{n_{{N_2}}}::1:2$$
D
$${n_{CO}}:{n_{{N_2}}}::2:3$$
Answer :
$${n_{CO}}:{n_{{N_2}}}::2:3$$
242. Representing $$P, V$$ and $$T$$ as pressure, volume and temperature, which of the following is the correct representation of Boyle's law ?
A
$$V \propto \frac{1}{T}\left( {P\,\,{\text{constant}}} \right)$$
B
$$V \propto \frac{1}{P}\left( {T\,\,{\text{constant}}} \right)$$
C
$$PV = RT$$
D
$$PV = nRT$$
Answer :
$$V \propto \frac{1}{P}\left( {T\,\,{\text{constant}}} \right)$$
243.
Plot of Maxwell's distribution of velocities is given below :
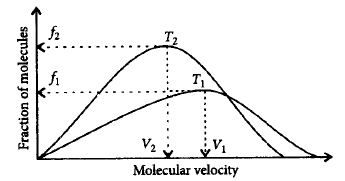
Which of the following is correct about this plot ?
A
$${T_2} > {T_1};{V_1} > {V_2};{f_1} > {f_2}$$
B
$${T_1} > {T_2};{V_1} < {V_2};{f_1} > {f_2}$$
C
$${T_1} < {T_2};{V_1} > {V_2};{f_2} > {f_1}$$
D
$${T_1} > {T_2};{V_1} > {V_2};{f_2} > {f_1}$$
Answer :
$${T_1} > {T_2};{V_1} > {V_2};{f_2} > {f_1}$$
244. Internal energy and pressure of a gas per unit volume are related as
A
$$p = \frac{2}{3}E$$
B
$$p = \frac{3}{2}E$$
C
$$p = \frac{1}{2}E$$
D
$$p = 2E$$
Answer :
$$p = \frac{2}{3}E$$
245. To which of the followings the Dalton's law of partial pressures is not applicable ?
A
$${H_2}\,\,{\text{and}}\,\,He$$
B
$$N{H_3}\,\,{\text{and}}\,\,HCl$$
C
$${N_2}\,\,{\text{and}}\,\,He$$
D
$$Xe\,\,{\text{and}}\,\,{O_2}$$
Answer :
$$N{H_3}\,\,{\text{and}}\,\,HCl$$
246. A compound formed by elements $$A$$ and $$B$$ crystallises in the cubic structure, where $$A$$ atoms are present at the corners of a cube and $$B$$ atoms are present at the face centres. The formula of the compound is
A
$${A_2}{B_2}$$
B
$$A{B_3}$$
C
$$AB$$
D
$${A_3}B$$
Answer :
$$A{B_3}$$
247. The surface tension of which of the following liquids is maximum ?
A
$${H_2}O$$
B
$${C_6}{H_6}$$
C
$$C{H_3}OH$$
D
$${C_2}{H_5}OH$$
Answer :
$${H_2}O$$
248. A $$10\,L$$ flask contains a gaseous mixture of $$CO$$ and $$C{O_2}$$ at a total pressure of $$2\,atm$$ and $$298\,K.$$ If $$0.20\,mole$$ of $$CO$$ is present, then its partial pressure is
A
$$0.49\,atm$$
B
$$1.51\,atm$$
C
$$1\,atm$$
D
$$2\,atm$$
Answer :
$$0.49\,atm$$
249.
The main assumptions of kinetic theory responsible for deviation of gases from ideal behaviour are
(i) there is no force of attraction between the molecules of a gas
(ii) volume of the molecules of a gas is negligibly small in comparison to the volume of the gas
(iii) particles of a gas are always in constant random motion.
A
(i) and (ii)
B
(ii) and (iii)
C
(i), (ii) and (iii)
D
(iii) only
Answer :
(i) and (ii)
250. A $$He$$ atom at $$300$$ $$K$$ is released from the surface of the earth to travel upwards. Assuming that it undergoes no collision with other molecules, how high will it be before coming to the rest?
A
$$9.53\,m$$
B
$$95.3\,m$$
C
$$953\,m$$
D
$$9.53 \times {10^4}m$$
Answer :
$$9.53 \times {10^4}m$$