271. Which of the following statements does not describe Charles' law ?
A
The volume of a given amount of a gas at constant pressure varies directly to its absolute temperature.
B
For each degree change in temperature, the volume of a sample of a gas changes by the fraction $$\frac{1}{{273}}$$ of its volume at $$0{\,^ \circ }C.$$
C
All gases expand or contract by the same fraction of their volume at $$0{\,^ \circ }C$$ per degree change in temperature.
D
$${V_t} = {V_0}\left( {\frac{{273 - t}}{{273}}} \right)$$
Answer :
$${V_t} = {V_0}\left( {\frac{{273 - t}}{{273}}} \right)$$
272. In van der Waals equation of state of the gas law, the constant $$'b'$$ is a measure of
A
volume occupied by the molecules
B
intermolecular attraction
C
intermolecular repulsions
D
intermolecular collisions per unit volume
Answer :
volume occupied by the molecules
273. Under what conditions gases generally deviate from ideal behaviour ?
A
At high temperature and low pressure
B
At low temperature and high pressure
C
At high temperature and high pressure
D
At low temperature and low pressure
Answer :
At low temperature and high pressure
274.
Study the figures given below and identify the type of interaction between $$XY - XY$$ molecules.
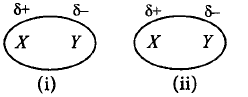
A
Dipole-Induced dipole
B
Dipole-Dipole
C
Dispersion forces
D
Induced dipole-Induced dipole.
Answer :
Dipole-Dipole
275. At $${0^ \circ }C$$ and one atm pressure, a gas occupies 100 $$cc.$$ If the pressure is increased to one and a half-time and temperature is increased by onethird of absolute temperature, then final volume of the gas will be :
A
80$$\,cc$$
B
88.9$$\,cc$$
C
66.7$$\,cc$$
D
100$$\,cc$$
Answer :
88.9$$\,cc$$
276.
In case of $$CO$$ and $$C{H_4}$$ curve goes to minima then increases with increase in pressure but in case of $${H_2}$$ and $$He$$ the curve is linear because :
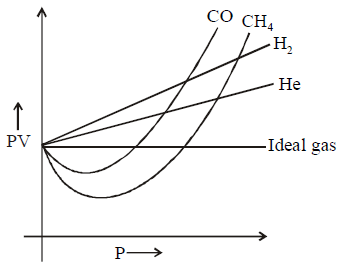
A
Intermolecular interactions for $${H_2}$$ and $$He$$ are very low.
B
Molecular size or atomic size for $${H_2}$$ and $$He$$ is small
C
Both (A) and (B)
D
Neither (A) nor (B)
Answer :
Both (A) and (B)
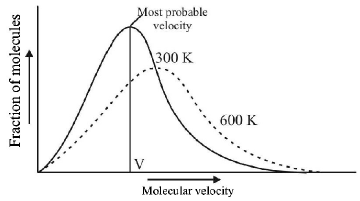
277. Which one of the following statements is $$NOT$$ true about the effect of an increase in temperature on the distribution of molecular speeds in a gas?
A
The area under the distribution curve remains the same as under the lower temperature
B
The distribution becomes broader
C
The fraction of the molecules with the most probable speed increases
D
The most probable speed increases
Answer :
The fraction of the molecules with the most probable speed increases
278. The number of atoms contained in a $$fcc$$ unit cell of a monoatomic substance is
A
1
B
2
C
4
D
6
Answer :
4