161. Which of the following systems will show Tyndall effect ?
A
Aqueous solution of sodium chloride
B
Aqueous solution of aluminium hydroxide
C
Aqueous solution of glucose
D
Aqueous solution of urea
Answer :
Aqueous solution of aluminium hydroxide
162. Which of the following is not correct for enzyme catalysis ?
A
The enzyme activity is maximum at optimum $$pH$$ which is between $$5{\text{ - }}7.$$
B
Each enzyme is specific for a given reaction.
C
The favourable temperature range of enzyme activity is between $$50{\text{ - }}60{\,^ \circ }C.$$
D
The enzymatic activity is increased in presence of certain substances called co-enzymes.
Answer :
The favourable temperature range of enzyme activity is between $$50{\text{ - }}60{\,^ \circ }C.$$
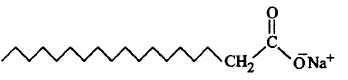
163.
In the given figure label the parts.

where 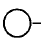 is $$CO{O^ - }$$
is $$CO{O^ - }$$
A
$$A$$ - Hydrophilic tail, $$B$$ - hydrophobic head
B
$$A$$ - Hydrophobic tail, $$B$$ - hydrophobic head
C
$$A$$ - Hydrophobic tail, $$B$$ - hydrophilic head
D
$$A$$ - Hydrophilic tail, $$B$$ - hydrophilic head
Answer :
$$A$$ - Hydrophobic tail, $$B$$ - hydrophilic head
164. Which of the following is most effective in causing the coagulation of ferric hydroxide sol?
A
$$KCl$$
B
$$KN{O_3}$$
C
$${K_2}S{O_4}$$
D
$${K_3}\left[ {Fe{{\left( {CN} \right)}_6}} \right]$$
Answer :
$${K_3}\left[ {Fe{{\left( {CN} \right)}_6}} \right]$$
165.
Which of the following statements are correct?
(i) A sol is prepared by addition of excess of $$AgN{O_3}$$ solution in $$KI$$ solution. The charge likely to develop on colloidal particle is positive.
(ii) The effect of pressure on physical adsorption is high if temperature is low.
(iii) Ultracentrifugation process is used for preparation of colloids.
(iv) Gold number is the index for extent of gold plating done.
A
(ii) and (iv) only
B
(i), (ii) and (iii) only
C
(i) and (ii) only
D
(i), (ii), (iii) and (iv)
Answer :
(i) and (ii) only
166. The coagulating power of electrolytes having ions $$N{a^ + }$$, $$A{l^{3 + }}$$ and $$B{a^{2 + }}$$ for arsenic sulphide sol increases in the order :
A
$$A{l^{3 + }} < B{a^{2 + }} < N{a^ + }$$
B
$$N{a^ + } < B{a^{2 + }} < A{l^{3 + }}$$
C
$$B{a^{2 + }} < N{a^ + } < A{l^{3 + }}$$
D
$$A{l^{3 + }} < N{a^ + } < B{a^{2 + }}$$
Answer :
$$N{a^ + } < B{a^{2 + }} < A{l^{3 + }}$$
167. Which one of the following statements is not correct in respect of lyophilic sols ?
A
There is a considerable interaction between the dispersed phase and dispersion medium
B
These are quite stable and are not easily coagulated
C
They carry charge
D
The particles are hydrated
Answer :
They carry charge
168. In an experiment, $$200\,mL$$ of $$0.5\,M$$ oxalic acid is shaken with $$10\,g$$ of activated charcoal and filtered. The concentration of the filtrate is reduced to $$0.4\,M.$$ The amount of adsorption $$\left( {\frac{x}{m}} \right)$$ is
A
0.9
B
1.8
C
0.18
D
0.09
Answer :
0.18
169. Fog is a colloidal solution of
A
solid particles dispersed in gas
B
solid particles dispersed in a liquid
C
liquid particles dispersed in gas
D
gaseous particles dispersed in a liquid
Answer :
liquid particles dispersed in gas
170. The efficiency of an enzyme in catalysing a reaction is due to its capacity
A
to form a strong enzyme-substrate complex
B
to decrease the bond energies of substrate molecule
C
to change the shape of the substrate molecule
D
to lower the activation energy of the reaction
Answer :
to lower the activation energy of the reaction