41.
Which of the following is not the correct difference between lyophobic and lyophilic sols?

A
(a)
B
(b)
C
(c)
D
(d)
Answer :
(b)
42. Which of the following statements is incorrect regarding physissorptions?
A
More easily liquefiable gases are adsorbed readily.
B
Under high pressure it results into multi molecular layer on adsorbent surface.
C
Enthalpy of adsorption $$\left( {\Delta {H_{adsorption}}} \right)$$ is low and positive.
D
It occurs because of van der Waal’s forces.
Answer :
Enthalpy of adsorption $$\left( {\Delta {H_{adsorption}}} \right)$$ is low and positive.
43. Which of the following gas molecules have maximum value of enthalpy of physisorption?
A
$${C_2}{H_6}$$
B
$$Ne$$
C
$${H_2}O$$
D
$${H_2}$$
Answer :
$${H_2}O$$
44. The Langmuir adsorption isotherm is deduced by using the assumption that
A
the adsorption takes place in multilayers
B
the adsorption sites are equivalent in their ability to adsorb the particles
C
the heat of adsorption varies with coverage
D
the adsorbed molecules interact with each other
Answer :
the adsorption sites are equivalent in their ability to adsorb the particles
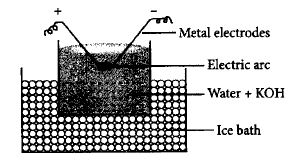
45.
In Bredig's arc method an electric arc is struck between the metal electrodes under the surface of water containing some stabilizing agent. The process involves
A
mechanical dispersion
B
condensation
C
both dispersion and condensation
D
ultrasonic dispersion
Answer :
both dispersion and condensation
46. At the high pressure, Langmuir adsorption isotherm takes the form
A
$$\frac{x}{m} = \frac{{ap}}{{1 + bp}}$$
B
$$\frac{x}{m} = \frac{a}{b}$$
C
$$\frac{x}{m} = ap$$
D
$$\frac{m}{x} = \frac{b}{a} + \frac{1}{{ap}}$$
Answer :
$$\frac{x}{m} = \frac{a}{b}$$
47. At the critical micelle concentration $$(CMC)$$ the surfactant molecules
A
decompose
B
dissociate
C
associate
D
become completely soluble
Answer :
associate
48. The term activation of adsorbent is used when
A
adsorbing power is increased by increasing surface area by making the surface rough
B
adsorbing power is increased by dipping the surface in acid to make it smooth
C
adsorbing power is increased by dissolving it in water
D
adsorbing power is decreased to reduce the extent of adsorption.
Answer :
adsorbing power is increased by increasing surface area by making the surface rough
49. $$Fe{\left( {OH} \right)_3}$$ sol can be more easily coagulated by $$N{a_3}P{O_4}$$ in comparison to $$KCl$$ because
A
mass of $$N{a_3}P{O_4}$$ is more than $$KCl$$ hence it is more effective than $$KCl.$$
B
phosphate ion $$\left( {PO_4^{3 - }} \right)$$ has higher negative charge than $$C{l^ - }$$ ion hence are more effective for coagulation.
C
$$KCl$$ is more soluble than $$N{a_3}P{O_4}$$ hence less effective for coagulation.
D
$$N{a^ + }$$ ions are more effective than $${K^ + }$$ ions for coagulation.
Answer :
phosphate ion $$\left( {PO_4^{3 - }} \right)$$ has higher negative charge than $$C{l^ - }$$ ion hence are more effective for coagulation.
50. Substances which behave as normal electrolytes in solution at low concentration and exhibit colloidal properties at higher concentration are called
A
lyophilic colloids
B
lyophobic colloids
C
macromolecular colloids
D
associated colloids
Answer :
associated colloids