51. Which of the following is an example of absorption?
A
Water on silica gel
B
Water on calcium chloride
C
Hydrogen on finely divided nickel
D
Oxygen on metal surface
Answer :
Water on calcium chloride
52.
The coagulation values in millimoles per litre of the electrolytes used for the coagulation of $$A{s_2}{S_3}$$ are given below
$$\eqalign{
& \left( {\text{i}} \right)\left( {NaCl} \right) = 52, \cr
& \left( {{\text{ii}}} \right)\left( {BaC{l_2}} \right) = 0.69 \cr
& \left( {{\text{iii}}} \right)\left( {MgS{O_4}} \right) = 0.22 \cr} $$
The correct order of their coagulating power is
A
$$\left( {\text{i}} \right) > \left( {{\text{ii}}} \right) > \left( {{\text{iii}}} \right)$$
B
$$\left( {{\text{ii}}} \right) > \left( {\text{i}} \right) > \left( {{\text{iii}}} \right)$$
C
$$\left( {{\text{iii}}} \right) > \left( {{\text{ii}}} \right) > \left( {\text{i}} \right)$$
D
$$\left( {{\text{iii}}} \right) > \left( {\text{i}} \right) > \left( {{\text{ii}}} \right)$$
Answer :
$$\left( {{\text{iii}}} \right) > \left( {{\text{ii}}} \right) > \left( {\text{i}} \right)$$
53.
The cause of Brownian movement which is not shown by true solutions or suspensions is due to
A
unbalanced bombardment of particles by molecules of the dispersion medium
B
attractive forces between dispersed phase and dispersion medium
C
larger size of the particles due to which they keep colliding and settling down
D
conversion currents formed in the sol.
Answer :
unbalanced bombardment of particles by molecules of the dispersion medium
54. Freshly prepared precipitate sometimes gets converted to colloidal solution by ______________.
A
coagulation
B
electrolysis
C
diffusion
D
peptisation
Answer :
peptisation
55. Which of the following graphs would yield a straight line?
A
$$\frac{x}{m}\,{\text{vs}}\,\,p$$
B
$$\log \,\frac{x}{m}\,\,{\text{vs}}\,\,p$$
C
$$\frac{x}{m}\,{\text{vs}}\,\,\log \,p$$
D
$$\log \,\frac{x}{m}\,\,{\text{vs}}\,\,\log \,p$$
Answer :
$$\log \,\frac{x}{m}\,\,{\text{vs}}\,\,\log \,p$$
56.
Which of the processes is being shown in the figure ?
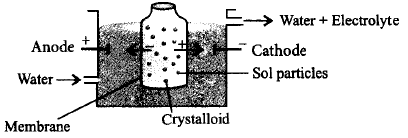
A
Electrodialysis
B
Electrokinetic
C
Electroosmosis
D
Electrophoresis
Answer :
Electrodialysis
57. The formation of miceiles takes place only above
A
critical temperature
B
Kraft temperature
C
inversion temperature
D
absolute temperature
Answer :
Kraft temperature
58. Under the influence of an electric field, the particles in a sol migrate towards cathode. The coagulation of the same sol is studied using $$NaCl,N{a_2}S{O_4}$$ and $$N{a_3}P{O_4}$$ solutions. Their coagulating values will be in the order
A
$$NaCl > N{a_2}S{O_4} > N{a_3}P{O_4}$$
B
$$N{a_2}S{O_4} > N{a_3}P{O_4} > NaCl$$
C
$$N{a_3}P{O_4} > N{a_2}S{O_4} > NaCl$$
D
$$N{a_2}S{O_4} > NaCl > N{a_3}P{O_4}$$
Answer :
$$NaCl > N{a_2}S{O_4} > N{a_3}P{O_4}$$
59. Which of the following process is not responsible for the presence of electric charge on the sol particles?
A
Electron capture by sol particles
B
Adsorption of ionic species from solution
C
Formation of Helmholtz electrical double layer
D
Absorption of ionic species from solution
Answer :
Absorption of ionic species from solution
60. Which one of the following is not applicable to the phenomenon of adsorption?
A
$$\Delta H > 0$$
B
$$\Delta G < 0$$
C
$$\Delta S < 0$$
D
$$\Delta H < 0$$
Answer :
$$\Delta H > 0$$