41. An experiment takes 10 minutes to raise the temperature of water in a container from $${0^ \circ }C$$ to $${100^ \circ }C$$ and another 55 minutes to convert it totally into steam by a heater supplying heat at a uniform rate. Neglecting the specific heat of the container and taking specific heat of water to be $$1\,cal/{g^ \circ }C,$$ the heat of vapourization according to this experiment will come out to be :
A
$$560\,cal/g$$
B
$$550\,cal/g$$
C
$$540\,cal/g$$
D
$$530\,cal/g$$
Answer :
$$550\,cal/g$$
42.
A student takes $$50\,gm$$ wax (specific heat $$ = 0.6\,kcal/k{g^ \circ }C$$ ) and heats it till it boils. The graph between temperature and time is as follows. Heat supplied to the wax per minute and boiling point are respectively
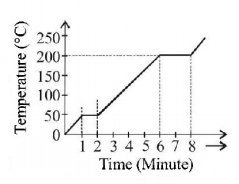
A
$$500\,cal,{50^ \circ }C$$
B
$$1000\,cal,{100^ \circ }C$$
C
$$1500\,cal,{200^ \circ }C$$
D
$$1000\,cal,{200^ \circ }C$$
Answer :
$$1500\,cal,{200^ \circ }C$$