91. Two containers $$A$$ and $$B$$ are partly filled with water and closed. The volume of $$A$$ is twice that of $$B$$ and it contains half the amount of water in $$B.$$ If both are at the same temperature, the water vapour in the containers will have pressure in the ratio of
A
$$1:2$$
B
$$1:1$$
C
$$2:1$$
D
$$4:1$$
Answer :
$$1:1$$
92. A balloon contains $$1500\,{m^3}$$ of helium at $${27^ \circ }C$$ and $$4$$ atmospheric pressure. The volume of helium at $$ - {3^ \circ }C$$ temperature and $$2$$ atmospheric pressure will,
A
$$1500\,{m^3}$$
B
$$1700\,{m^3}$$
C
$$1900\,{m^3}$$
D
$$2700\,{m^3}$$
Answer :
$$2700\,{m^3}$$
93. The average translational kinetic energy of $${O_2}$$ (relative molar mass 32) molecules at a particular temperature is $$0.048\,eV.$$ The translational kinetic energy of $${N_2}$$ (relative molar mass 28) molecules in $$eV$$ at the same temperature is
A
0.0015
B
0.003
C
0.048
D
0.768
Answer :
0.048
94. Two gases occupy two containers $$A$$ and $$B$$ the gas in $$A,$$ of volume $$0.10\,{m^3},$$ exerts a pressure of $$1.40\,MPa$$ and that in $$B$$ of volume $$0.15\,{m^3}$$ exerts a pressure $$0.7\,MPa.$$ The two containers are united by a tube of negligible volume and the gases are allowed to intermingle. Then if the temperature remains constant, the final pressure in the container will be (in $$MPa$$ )
A
0.70
B
0.98
C
1.40
D
210
Answer :
0.98
95. If for a gas, $$\frac{R}{{{C_v}}} = 0.67,$$ the gas is made up of molecules which are
A
diatomic
B
mixture of diatomic and polyatomic molecules
C
monoatomic
D
polyatomic
Answer :
monoatomic
96.
The figure shows graph of pressure and volume of a gas at two different temperatures $${T_1}$$ and $${T_2}.$$ Which of the following inferences is correct?
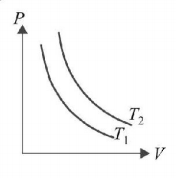
A
$${T_1} > {T_2}$$
B
$${T_1} = {T_2}$$
C
$${T_1} < {T_2}$$
D
None of these
Answer :
$${T_1} < {T_2}$$
97. $$N\left( { < 100} \right)$$ molecules of a gas have velocities $$1,2,3,\,.........N\,km/s$$ respectively. Then ratio of $$rms$$ speed and average speed is
A
1
B
$$\frac{{\sqrt {\left( {2N + 1} \right)\left( {N + 1} \right)} }}{{6N}}$$
C
$$\frac{{\sqrt {\left( {2N + 1} \right)\left( {N + 1} \right)} }}{6}$$
D
$$2\sqrt {\frac{{2N + 1}}{{6\left( {N + 1} \right)}}} $$
Answer :
$$2\sqrt {\frac{{2N + 1}}{{6\left( {N + 1} \right)}}} $$
98. In an ideal gas at temperature $$T,$$ the average force that a molecule applies on the walls of a closed container depends on $$T$$ as $${T^q}.$$ A good estimate for $$q$$ is:
A
$$\frac{1}{2}$$
B
$$2$$
C
$$1$$
D
$$\frac{1}{4}$$
Answer :
$$1$$
99. Relation between pressure $$\left( P \right)$$ and energy $$\left( E \right)$$ of a gas is
A
$$P = \frac{2}{3}E$$
B
$$P = \frac{1}{3}E$$
C
$$P = \frac{1}{2}E$$
D
$$P = 3E$$
Answer :
$$P = \frac{2}{3}E$$
100.
The density $$\left( \rho \right)$$ versus pressure $$\left( P \right)$$ of a given mass of an ideal gas is shown at two temperatures $${T_1}$$ and $${T_2}$$ Then relation between $${T_1}$$ and $${T_2}$$ may be
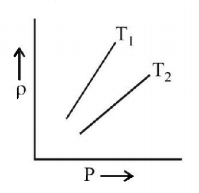
A
$${T_1} > {T_2}$$
B
$${T_2} > {T_1}$$
C
$${T_1} = {T_2}$$
D
All the three are possible
Answer :
$${T_2} > {T_1}$$