101.
Starting with the same initial conditions, an ideal gas expands from volume $${V_1}$$ to $${V_2}$$ in three different ways. The work done by the gas is $${W_1}$$ if the process is purely isothermal, $${W_2}$$ if purely isobaric and $${W_3}$$ if purely adiabatic. Then
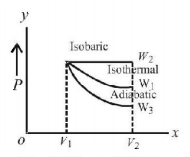
A
$${W_2} > {W_1} > {W_3}$$
B
$${W_2} > {W_3} > {W_1}$$
C
$${W_1} > {W_2} > {W_3}$$
D
$${W_1} > {W_3} > {W_2}$$
Answer :
$${W_2} > {W_1} > {W_3}$$
102. If for a gas, $$\frac{R}{{{C_V}}} = 0.67,$$ this gas is made up of molecules which are
A
diatomic
B
mixture of diatomic and polyatomic molecules
C
monoatomic
D
polyatomic
Answer :
monoatomic
103.
A thermodynamic system is taken from state $$A$$ to $$B$$ along $$ACB$$ and is brought back to $$A$$ along $$BDA$$ as shown in the $$p-V$$ diagram. The net work done during the complete cycle is given by the area
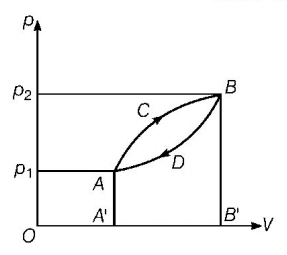
A
$${p_1}ACB{p_2}{p_1}$$
B
$$ACBB'A'A$$
C
$$ACBDA$$
D
$$ADBB'A'A$$
Answer :
$$ACBDA$$
104. A Centigrade and a Fahrenheit thermometer are dipped in boiling water. The water temperature is lowered until the Fahrenheit thermometer registers $${140^ \circ }.$$ What is the fall in temperature as registered by the Centigrade thermometer?
A
$${80^ \circ }$$
B
$${60^ \circ }$$
C
$${40^ \circ }$$
D
$${30^ \circ }$$
Answer :
$${40^ \circ }$$
105. The work done in an adiabatic change in a particular gas depends only upon
A
change in volume
B
change in temperature
C
change in pressure
D
None of these
Answer :
change in temperature
106.
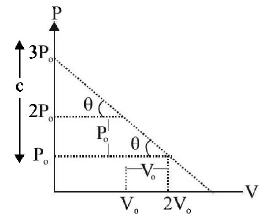
$$'n'$$ moles of an ideal gas undergoes a process $$A \,\,®\,\, B$$ as shown in the figure. The maximum temperature of the gas during the process will be:
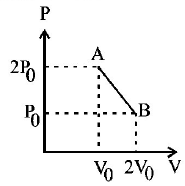
A
$$\frac{{9\,{P_0}{V_0}}}{{2\,nR}}$$
B
$$\frac{{9\,{P_0}{V_0}}}{{nR}}$$
C
$$\frac{{9\,{P_0}{V_0}}}{{4\,nR}}$$
D
$$\frac{{3\,{P_0}{V_0}}}{{2\,nR}}$$
Answer :
$$\frac{{9\,{P_0}{V_0}}}{{4\,nR}}$$
107. During an adiabatic process an object does $$100\,J$$ of work and its temperature decreases by $$5K.$$ During another process it does $$25\,J$$ of work and its temperature decreases by $$5K.$$ Its heat capacity for $${2^{nd}}$$ process is
A
$$20\,J/K$$
B
$$24\,J/K$$
C
$$15\,J/K$$
D
$$100\,J/K$$
Answer :
$$15\,J/K$$
108.
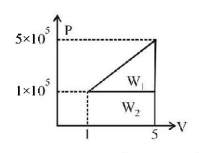
A system changes from the state $$\left( {{P_1},{V_1}} \right)$$ to $$\left( {{P_2},{V_2}} \right)$$ as shown in the figure. What is the work done by the system?
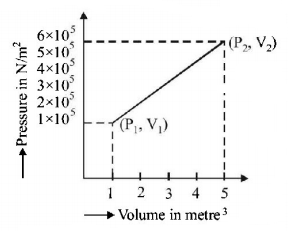
A
$$7.5 \times {10^5}\,joule$$
B
$$7.5 \times {10^5}\,erg$$
C
$$12 \times {10^5}\,joule$$
D
$$6 \times {10^5}\,joule$$
Answer :
$$12 \times {10^5}\,joule$$
109. A Carnot engine takes $$3 \times {10^6}{{\,cal}}{\text{.}}$$ of heat from a reservoir at $${27^ \circ }C,$$ and gives it to a sink at $${27^ \circ }C.$$ The work done by the engine is
A
$$4.2 \times {10^6}\,J$$
B
$$8.4 \times {10^6}\,J$$
C
$$16.8 \times {10^6}\,J$$
D
zero
Answer :
$$8.4 \times {10^6}\,J$$
110. Even Carnot engine cannot give 100% efficiency because we cannot
A
prevent radiation
B
find ideal sources
C
reach absolute zero temperature
D
eliminate friction.
Answer :
reach absolute zero temperature