31. Some of the thermodynamic parameters are state variables while some are process variables. Some grouping of the parameters are given. Choose the correct one
A
State variables : Temperature, no. of moles
Process variables : Internal energy, work done by the gas
Process variables : Internal energy, work done by the gas
B
State variables : Volume, temperature
Process variables : Internal energy, work done by the gas
Process variables : Internal energy, work done by the gas
C
State variables : work done by the gas, heat rejected by the gas.
Process variables : Temperature, volume
Process variables : Temperature, volume
D
State variables : Internal energy, volume
Process variables : Work done by the gas, heat absorbed by the gas
Process variables : Work done by the gas, heat absorbed by the gas
Answer :
State variables : Internal energy, volume
Process variables : Work done by the gas, heat absorbed by the gas
Process variables : Work done by the gas, heat absorbed by the gas
32. Which of the following graphs correctly represents the variation of $$\beta = - \frac{{\frac{{dV}}{{dP}}}}{V}$$ with $$P$$ for an ideal gas at constant temperature?
A
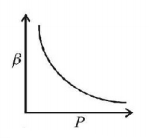

B
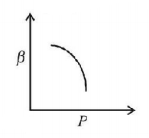

C
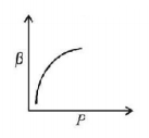

D
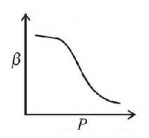

Answer :


33. We consider a thermodynamic system. If $$\Delta U$$ represents the increase in its internal energy and $$W$$ the work done by the system, which of the following statements is true ?
A
$$\Delta U = - W$$ in an adiabatic process
B
$$\Delta U = W$$ in an isothermal process
C
$$\Delta U = - W$$ in an isothermal process
D
$$\Delta U = W$$ in an adiabatic process
Answer :
$$\Delta U = - W$$ in an adiabatic process
34. If $${C_P}$$ and $${C_V}$$ denote the specific heats of nitrogen per unit mass at constant pressure and constant volume respectively, then
A
$${C_P} - {C_V} = 28\,R$$
B
$${C_P} - {C_V} = \frac{R}{{28}}$$
C
$${C_P} - {C_V} = \frac{R}{{14}}$$
D
$${C_P} - {C_V} = R$$
Answer :
$${C_P} - {C_V} = \frac{R}{{28}}$$
35. Which of the following is incorrect regarding the first law of thermodynamics ?
A
It is a restatement of the principle of conservation of
energy
B
It is not applicable to any cyclic process
C
It introduces the concept of the entropy
D
It introduces the concept of the internal energy
Answer :
It is not applicable to any cyclic process
36. An ideal gas at $${27^ \circ }C$$ is compressed adiabatically to $$\frac{8}{{27}}$$ of its original volume. The rise in temperature is $$\left( {\gamma = \frac{5}{3}} \right)$$
A
$${475^ \circ }C$$
B
$${402^ \circ }C$$
C
$${275^ \circ }C$$
D
$${375^ \circ }C$$
Answer :
$${375^ \circ }C$$
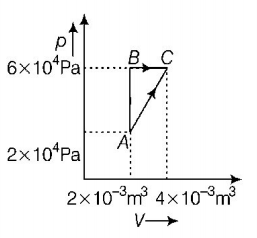
37.
Figure below shows two paths that may be taken by a gas to go from a state $$A$$ to a state $$C.$$
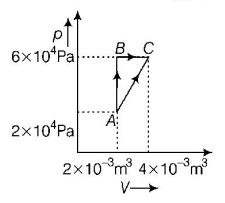
In process $$AB,400\,J$$ of heat is added to the system and in process $$BC,100\,J$$ of heat is added to the system. The heat absorbed by the system in the process $$AC$$ will be
A
$$380\,J$$
B
$$500\,J$$
C
$$460\,J$$
D
$$300\,J$$
Answer :
$$460\,J$$
38. A Carnot engine, whose efficiency is 40%, takes in heat from a source maintained at a temperature of 500$$K.$$ It is desired to have an engine of efficiency 60%. Then, the intake temperature for the same exhaust $$(sin\,k)$$ temperature must be :
A
efficiency of Carnot engine cannot be made larger than
50%
B
1200 $$K$$
C
750 $$K$$
D
600 $$K$$
Answer :
750 $$K$$
39. An ideal Carnot engine, whose efficiency is $$40\% $$ receives heat at $$500\,K.$$ If its efficiency is $$50\% ,$$ then the intake temperature for the same exhaust temperature is
A
$$600\,K$$
B
$$700\,K$$
C
$$800\,K$$
D
$$900\,K$$
Answer :
$$600\,K$$
40.
A $$500\,ml$$ sealed cylinder contains nitrogen at a pressure of $$1\,atm.$$ A tiny glass tube lies at the bottom of the cylinder. Its volume is $$0.50\,ml$$ and it contains hydrogen at a pressure of $$4.5\,atm.$$ The glass tube is broken so that hydrogen also fills the cylinder. The new pressure in the cylinder is
$$\left( {1\,atm = 1 \times {{10}^5}\,N/{m^2}} \right)$$
A
$$76.34\,cm\,Hg$$
B
$$82.40\,cm\,Hg$$
C
$$94.24\,cm\,Hg$$
D
$$104.34\,cm\,Hg$$
Answer :
$$76.34\,cm\,Hg$$