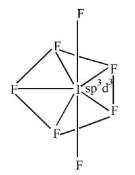
191. The structure of $$I{F_7}$$ is
A
square pyramidal
B
trigonal bipyramidal
C
octahedral
D
pentagonal bipyramidal
Answer :
pentagonal bipyramidal
192. Which structure is linear?
A
$$S{O_2}$$
B
$$C{O_2}$$
C
$$CO_3^{2 - }$$
D
$$SO_4^{2 - }$$
Answer :
$$C{O_2}$$
193. Which of the following molecular species has unpaired electron(s) ?
A
$${N_2}$$
B
$${F_2}$$
C
$$O_2^ - $$
D
$$O_2^{2 - }$$
Answer :
$$O_2^ - $$
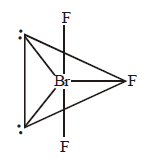
194. In $$Br{F_3}$$ molecule, the lone pairs occupy equatorial positions to minimize
A
lone pair - bond pair repulsion only
B
bond pair - bond pair repulsion only
C
lone pair - lone pair repulsion and lone pair - bond pair repulsion
D
lone pair - lone pair repulsion only
Answer :
lone pair - lone pair repulsion and lone pair - bond pair repulsion
195. The group of molecules having identical shape is :
A
$$PC{l_5},I{F_5},Xe{O_2}{F_2}$$
B
$$B{F_3},PC{l_3},Xe{O_3}$$
C
$$S{F_4},Xe{F_4},CC{l_4}$$
D
$$Cl{F_4},XeO{F_2},XeF_3^ + $$
Answer :
$$Cl{F_4},XeO{F_2},XeF_3^ + $$
196. Among the following, the molecule that is linear is
A
$$\,C{O_2}$$
B
$$N{O_2}$$
C
$$S{O_2}$$
D
$$\,Cl{O_2}$$
Answer :
$$\,C{O_2}$$
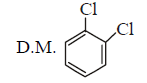
197. Dipole moment is shown by
A
1, 4 - dichlorobenzene
B
$$cis$$ - 1, 2 - dichlorobenzene
C
$$trans$$ - 1, 3 - dichlorobenzene
D
$$trans$$ - 2, 3 - dichloro - 2 - butene
Answer :
$$cis$$ - 1, 2 - dichlorobenzene
198. Which one shows maximum hydrogen bonding?
A
$${H_2}O$$
B
$${H_2}Se$$
C
$${H_2}S$$
D
$$HF$$
Answer :
$$HF$$
199. In which of the following molecules octet rule is not followed?
A
$$N{H_3}$$
B
$$C{H_4}$$
C
$$C{O_2}$$
D
$$NO$$
Answer :
$$NO$$
200. What is the correct dipole moment of $$N{H_3}$$ and $$N{F_3}$$ respectively?
A
$$4.90 \times {10^{ - 30}}\,C\,m\,\,{\text{and}}$$ $${\text{0}}{\text{.80}} \times {10^{ - 30}}\,C\,m$$
B
$$0.80 \times {10^{ - 30}}\,C\,m\,\,{\text{and}}$$ $${\text{4}}{\text{.90}} \times {10^{ - 30}}\,C\,m$$
C
$$4.90 \times {10^{ - 30}}\,C\,m\,\,{\text{and}}$$ $${\text{4}}{\text{.90}} \times {10^{ - 30}}\,C\,m$$
D
$$0.80 \times {10^{ - 30}}\,C\,m\,\,{\text{and}}$$ $${\text{0}}{\text{.80}} \times {10^{ - 30}}\,C\,m$$
Answer :
$$4.90 \times {10^{ - 30}}\,C\,m\,\,{\text{and}}$$ $${\text{0}}{\text{.80}} \times {10^{ - 30}}\,C\,m$$