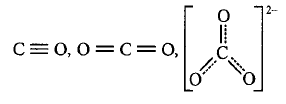251. Which of the following does not apply to metallic bond?
A
Overlapping valence orbitals
B
Mobile valence electrons
C
Delocalised electrons
D
Highly directed bonds
Answer :
Highly directed bonds
252. The correct order of $$N - O$$ bond lengths in $$NO,NO_2^ - ,NO_3^ - $$ and $${N_2}{O_4}$$ is
A
$${N_2}{O_4} > NO_2^ - > NO_3^ - \, > NO$$
B
$$NO > NO_3^ - \, > {N_2}{O_4} > NO_2^ - $$
C
$$NO_3^ - \, > NO_2^ - > {N_2}{O_4} > NO$$
D
$$NO > {N_2}{O_4} > NO_2^ - > NO_3^ - $$
Answer :
$$NO_3^ - \, > NO_2^ - > {N_2}{O_4} > NO$$
253.
Pick out the isoelectronic structures from the following;
$$\eqalign{
& \left( {\text{i}} \right)CH_3^ + \cr
& \left( {{\text{ii}}} \right){H_3}{O^ + } \cr
& \left( {{\text{iii}}} \right)N{H_3} \cr
& \left( {{\text{iv}}} \right)CH_3^ - \cr} $$
A
(i) and (ii)
B
(iii) and (iv)
C
(i) and (iii)
D
(ii), (iii) and (iv)
Answer :
(ii), (iii) and (iv)
254. Bond order of $$N_2^ + ,N_2^ - $$ and $${N_2}$$ will be
A
2.5, 2.5 and 3 respectively
B
2, 2.5 and 3 respectively
C
3, 2.5 and 3 respectively
D
2.5, 2.5 and 2.5 respectively
Answer :
2.5, 2.5 and 3 respectively
255. Which of the following elements forms predominantly covalent compounds as compared to other elements which form ionic compounds?
A
$$Be$$
B
$$Mg$$
C
$$Ca$$
D
$$Sr$$
Answer :
$$Be$$
256. The hybridisation of atomic orbitals of nitrogen in $$NO_2^ + ,NO_3^ - $$ and $$NH_4^ + $$ are
A
$$sp,s{p^3}$$ and $$s{p^2}$$ respectively
B
$$sp,s{p^2}$$ and $$s{p^3}$$ respectively
C
$$s{p^2},sp$$ and $$s{p^3}$$ respectively
D
$$s{p^2},s{p^3}$$ and $$sp$$ respectively
Answer :
$$sp,s{p^2}$$ and $$s{p^3}$$ respectively
257. The hybridisation of sulphur in sulphur dioxide is :
A
$$sp$$
B
$$s{p^3}$$
C
$$s{p^2}$$
D
$$ds{p^2}$$
Answer :
$$s{p^2}$$
258.
The ground state electronic configuration of valence shell electrons in nitrogen molecule $$\left( {{N_2}} \right)$$ is written as $$KK,\sigma 2{s^2},\mathop \sigma \limits^* 2{s^2},\sigma 2p_x^2,\pi 2p_y^2$$ $$ \approx \pi 2p_z^2$$
Bond order in nitrogen molecule is
A
0
B
1
C
2
D
3
Answer :
3
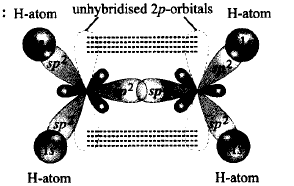
259. In formation of ethene, the bond formation between $$s$$ and $$p$$ - orbitals takes place in which of the following manner?
A
$$s{p^2}$$ hybridised orbitals form sigma bond while the unhybridised $$\left( {{p_x}\,\,{\text{or}}\,\,{p_y}} \right)$$ overlaps sidewise to form $$\pi $$ - bond.
B
$$s{p^2}$$ hybridised orbitals form $$\pi $$ - bond while the unhybridised $$\left( {{p_z}} \right)$$ overlaps to form $$\sigma $$ - bond.
C
$$s{p^2}$$ hybridised orbitals overlap with $$s$$ - orbitals of $$H$$ atoms while unhybridised orbitals form $$C-C$$ bond.
D
$$s{p^2}$$ hybridised orbitals form sigma bonds with $$H$$ atoms while unhybridised orbitals form $$\pi $$ - bonds between $$C$$ and $$H$$ atoms.
Answer :
$$s{p^2}$$ hybridised orbitals form sigma bond while the unhybridised $$\left( {{p_x}\,\,{\text{or}}\,\,{p_y}} \right)$$ overlaps sidewise to form $$\pi $$ - bond.
260. The correct order of decreasing bond lengths of $$CO,C{O_2}$$ and $$CO_3^{2 - }$$ is
A
$$CO > C{O_2} > CO_3^{2 - }$$
B
$$CO_3^{2 - } > C{O_2} > CO$$
C
$$C{O_2} > CO > CO_3^{2 - }$$
D
$$C{O_2} > CO_3^{2 - } > CO$$
Answer :
$$CO_3^{2 - } > C{O_2} > CO$$