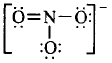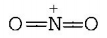271. Which of the following statement is not correct for sigma and $$pi - {\text{bonds}}$$ formed between two carbon atoms?
A
Free rotation of atoms about a sigma bond is allowed but not in case of a $$pi - {\text{bond}}$$
B
Sigma bond determines the direction between carbon atoms but a $$pi - {\text{bond}}$$ has no primary effect in this regard
C
Sigma bond is stronger than a $$pi - {\text{bond}}$$
D
Bond energies of sigma and $$pi - {\text{bonds}}$$ are of the order of $$264\,kJ/mol$$ and $$347\,kJ/mol,$$ respectively
Answer :
Bond energies of sigma and $$pi - {\text{bonds}}$$ are of the order of $$264\,kJ/mol$$ and $$347\,kJ/mol,$$ respectively
272. Which of the following molecule does not have a linear arrangement of atoms?
A
$${H_2}S$$
B
$${C_2}{H_2}$$
C
$$Be{H_2}$$
D
$$C{O_2}$$
Answer :
$${H_2}S$$
273. In $$NO_3^ - $$ ion, the number of bond pairs and lone pairs of electrons on nitrogen atom are
A
2, 2
B
3, 1
C
1, 3
D
4, 0
Answer :
4, 0
274. Main axis of a diatomic molecule is $$z$$ molecular orbital, $${p_x}$$ and $${p_y}$$ overlaps to form which of the following orbitals?
A
$$\pi - $$ molecular orbital
B
$$\sigma - $$ molecular orbital
C
$$\delta - $$ molecular orbital
D
No bond will form
Answer :
$$\pi - $$ molecular orbital
275. Linus Pauling received the Nobel Prize for his work on
A
atomic structure
B
photosynthesis
C
chemical bonds
D
thermodynamics
Answer :
chemical bonds
276. Which of the following species has a linear shape?
A
$$NO_2^ - $$
B
$$S{O_2}$$
C
$$NO_2^ + $$
D
$${O_3}$$
Answer :
$$NO_2^ + $$
277.
For which of the following molecule significant $$\mu \ne 0?$$
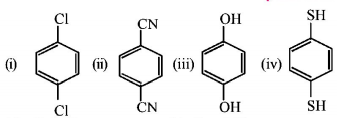
A
Only (i)
B
(i) and (ii)
C
Only (iii)
D
(iii) and (iv)
Answer :
(iii) and (iv)
278. Which of the following formulae does not show the correct relationship?
A
$$B.O. = \frac{1}{2}\left( {{N_b} - {N_a}} \right)$$
B
$$B.O. \propto \frac{1}{{{\text{Bond}}\,\,{\text{length}}}}$$
C
$$B.O. \propto \frac{1}{{{\text{Bond dissociation energy}}}}$$
D
$${N_b} > {N_a},B.O. = + ve$$
Answer :
$$B.O. \propto \frac{1}{{{\text{Bond dissociation energy}}}}$$
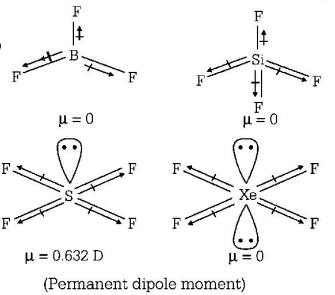
279. Which of the following would have a permanent dipole moment?
A
$$B{F_3}$$
B
$$Si{F_4}$$
C
$$S{F_4}$$
D
$$Xe{F_4}$$
Answer :
$$S{F_4}$$
280. Number of paired electrons in $${O_2}$$ molecule is :
A
7
B
8
C
16
D
14
Answer :
14