1. The highest value of the calculated spin only magnetic moment ( in $$BM$$ ) among all the transition metal complexes is:
A
5.92
B
6.93
C
3.87
D
4.90
Answer :
5.92
2. The coordination number and the oxidation state of the element $$'E'$$ in the complex $$\left[ {E{{\left( {en} \right)}_2}\left( {{C_2}{O_4}} \right)} \right]N{O_2}$$ (where $$\left( {en} \right)$$ is ethylene diamine) are, respectively,
A
6 and 2
B
4 and 2
C
4 and 3
D
6 and 3
Answer :
6 and 3
3. The correct statement about the magnetic properties of $${\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{3 - }}$$ and $${\left[ {Fe{F_6}} \right]^{3 - }}$$ is $$\left( {Z = 26} \right):$$
A
both are paramagnetic.
B
both are diamagnetic.
C
$${\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{3 - }}$$ is diamagnetic, $${\left[ {Fe{F_6}} \right]^{3 - }}$$ is paramagnetic.
D
$${\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{3 - }}$$ is paramagnetic, $${\left[ {Fe{F_6}} \right]^{3 - }}$$ is diamagnetic.
Answer :
both are paramagnetic.
4. The number of ions given by $$\left[ {Pt{{\left( {N{H_3}} \right)}_6}} \right]C{l_4}$$ in aqueous solution will be
A
two
B
three
C
five
D
eleven
Answer :
five
5. $$AgCl$$ is soluble in $$N{H_4}OH$$ solution. The solubility is due to formation of
A
$$AgOH$$
B
$$A{g_2}O$$
C
$${\left[ {Ag{{\left( {N{H_3}} \right)}_2}} \right]^ + }$$
D
$$N{H_4}Cl$$
Answer :
$${\left[ {Ag{{\left( {N{H_3}} \right)}_2}} \right]^ + }$$
6. Among the following which is not the $$\pi $$ - bonded organometallic compound ?
A
$$K\left[ {PtC{l_3}\left( {{\eta ^2} - {C_2}{H_4}} \right)} \right]$$
B
$$Fe{\left( {{\eta ^5} - {C_5}{H_5}} \right)_2}$$
C
$$Cr{\left( {{\eta ^6} - {C_6}{H_6}} \right)_2}$$
D
$${\left( {C{H_3}} \right)_4}Sn$$
Answer :
$${\left( {C{H_3}} \right)_4}Sn$$
7. Which of the following statements is true?
A
$$\left[ {Ni{{\left( {CO} \right)}_4}} \right]$$ is high spin complex.
B
Weak ligands like $${F^ - },C{l^ - }$$ and $$O{H^ - }$$ usually form low spin complexes.
C
$${\left[ {Fe{F_6}} \right]^{3 - }}$$ is a high spin complex .
D
Strong ligand like $$C{N^ - }$$ and $$NO_2^ - ,$$ generally form high spin complexes.
Answer :
$${\left[ {Fe{F_6}} \right]^{3 - }}$$ is a high spin complex .
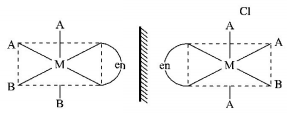
8.
The one that will show optical activity is:
$$\left( {{\text{en }} = {\text{ethane }}1,{\text{ }}2 - {\text{diamine}}} \right){\text{ }}$$
A
.PNG)
.PNG)
B
.PNG)
.PNG)
C
.PNG)
.PNG)
D
.PNG)
.PNG)
Answer :
.PNG)
.PNG)
9. The correct increasing order of trans-effect of the following species is
A
$$N{H_3} > C{N^ - } > B{r^ - } > {C_6}H_5^ - $$
B
$$C{N^ - } > {C_6}H_5^ - > B{r^ - } > N{H_3}$$
C
$$B{r^ - } > C{N^ - } > N{H_3} > {C_6}H_5^ - $$
D
$$C{N^ - } > B{r^ - } > {C_6}H_5^ - > N{H_3}$$
Answer :
$$C{N^ - } > {C_6}H_5^ - > B{r^ - } > N{H_3}$$
10.
Which of the following statements is/are correct?
(i) In octahedral complexes, $${t_{2g}}$$ orbitals possess low energy as compared to $${e_g}$$ orbitals.
(ii) In tetrahedral complexes, $${t_2}$$ orbitals possess high energy as compared to $$e$$ orbitals.
(iii) In octahedral complexes, $${e_g}$$ orbitals possess low energy as compared to $${t_{2g}}$$ orbitals.
A
(ii) Only
B
(iii) Only
C
(i) and (ii)
D
(i) and (iii)
Answer :
(i) and (ii)