121.
Which one of the following complexes is an outer orbital complex?
$$\left( {{\text{Atomic}}\,{\text{nos}}.:Mn = 25;Fe = 26;Co = 27,Ni = 28} \right)$$
A
$${\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }}$$
B
$${\left[ {Mn{{\left( {CN} \right)}_6}} \right]^{4 - }}$$
C
$${\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{4 - }}$$
D
$${\left[ {Ni{{\left( {N{H_3}} \right)}_6}} \right]^{2 + }}$$
Answer :
$${\left[ {Ni{{\left( {N{H_3}} \right)}_6}} \right]^{2 + }}$$
122. The sum of coordination number and oxidation number of the metal $$M$$ in the complex $$\left[ {M{{\left( {en} \right)}_2}\left( {{C_2}{O_4}} \right)} \right]Cl$$ ( where, $$en$$ is ethylenediamine ) is
A
9
B
6
C
7
D
8
Answer :
6
123. A square planar complex is formed by hybridisation of which atomic orbitals?
A
$$s,{p_x},{p_y},{d_{yz}}$$
B
$$s,{p_x},{p_y},{d_{{x^2} - {y^2}}}$$
C
$$s,{p_x},{p_y},{d_{{z^2}}}$$
D
$$s,{p_y},{p_z},{d_{xy}}$$
Answer :
$$s,{p_x},{p_y},{d_{{x^2} - {y^2}}}$$
124. When excess of aqueous $$KCN$$ solution is added to an aqueous solution of copper sulphate, the complex $${\left[ {Cu{{\left( {CN} \right)}_4}} \right]^{2 - }}$$ is formed. On passing $${H_2}S$$ gas through this solution no precipitate of $$CuS$$ is formed because
A
sulphide ions cannot replace $$C{N^ - }$$ ions
B
$${\left[ {Cu{{\left( {CN} \right)}_4}} \right]^{2 - }}$$ does not give $$C{u^{2 + }}$$ ion in the solution
C
sulphide ions from $${H_2}S$$ do not form complexes
D
sulphide ions cannot replace sulphate ions from copper sulphate solution
Answer :
$${\left[ {Cu{{\left( {CN} \right)}_4}} \right]^{2 - }}$$ does not give $$C{u^{2 + }}$$ ion in the solution
125. $${\left[ {Fe{F_6}} \right]^{3 - }}$$ is paramagnetic due to presence of unpaired electrons in the complex. The five electrons remain unpaired because
A
fluorine is the most electronegative element
B
$${F^ - }$$ is a weak field ligand hence does not cause pairing of electrons
C
$${F^ - }$$ is a strong field ligand hence does not cause pairing of electrons
D
pairing does not take place in iron complexes
Answer :
$${F^ - }$$ is a weak field ligand hence does not cause pairing of electrons
126.
What type of isomerism exists in the following pairs of complexes?
$$\left( {\text{i}} \right)\left[ {Co{{\left( {N{H_3}} \right)}_5}N{O_3}} \right]S{O_4}\,\,{\text{and}}\,\,$$ $$\left[ {Co{{\left( {N{H_3}} \right)}_5}S{O_4}} \right]N{O_3}$$
$$\left( {{\text{ii}}} \right)\left[ {Co\left( {en} \right){{\left( {{H_2}O} \right)}_2}C{l_2}} \right]Cl\,\,{\text{and}}\,\,$$ $$\left[ {Co\left( {en} \right)\left( {{H_2}O} \right)C{l_3}} \right]{H_2}O$$
A
(i) Ionisation (ii) Hydrate
B
(i) Linkage (ii) Hydrate
C
(i) Ionisation (ii) Linkage
D
(i) Linkage (ii) Coordination
Answer :
(i) Ionisation (ii) Hydrate
127.
The diamagnetic species is :
$$\eqalign{
& \left( {\text{i}} \right){\left[ {Cu{{\left( {CN} \right)}_4}} \right]^{3 - }} \cr
& \left( {{\text{ii}}} \right){\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }} \cr
& \left( {{\text{iii}}} \right){\left[ {Ni{{\left( {N{H_3}} \right)}_6}} \right]^{2 + }} \cr
& \left( {{\text{iv}}} \right)\left[ {Fe{{\left( {CN} \right)}_6}} \right] \cr} $$
A
(i), (iii)
B
(i), (ii)
C
(iii), (iv)
D
only (iv)
Answer :
(i), (ii)
128. Consider the following complex $$\left[ {Co{{\left( {N{H_3}} \right)}_5}C{O_3}} \right]Cl{O_4}.$$ The coordination number, oxidation number, number of $$d$$ - electrons and number of unpaired $$d$$ - electrons on the metal are respectively
A
6, 3, 6, 0
B
7, 2, 7, 1
C
7, 1, 6, 4
D
6, 2, 7, 3
Answer :
6, 3, 6, 0
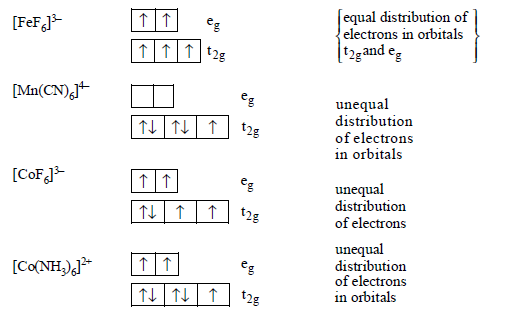
129. Which of the following complex ions has electrons that are symmetrically filled in both $${t_{2g}}$$ and $${e_g}$$ orbitals ?
A
$${\left[ {Fe{F_6}} \right]^{3 - }}$$
B
$${\left[ {Mn{{\left( {CN} \right)}_6}} \right]^{4 - }}$$
C
$${\left[ {Co{F_6}} \right]^{3 - }}$$
D
$${\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]^{2 + }}$$
Answer :
$${\left[ {Fe{F_6}} \right]^{3 - }}$$
130. Consider the coordination compound, $$\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]C{l_3}.$$ In the formation of this complex, the species which acts as the Lewis acid is :
A
$${\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }}$$
B
$$C{l^ - }$$
C
$$C{o^{3 + }}$$
D
$$N{H_3}$$
Answer :
$${\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }}$$