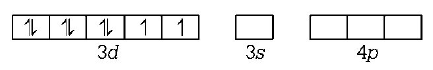
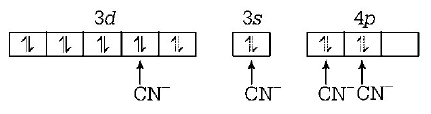
21. Which of the following does not depict the correct name of the compound?
A
$${K_2}\left[ {Zn{{\left( {OH} \right)}_4}} \right]:$$ Potassiumtetrahydroxidozincate(II)
B
$$\left[ {Co{{\left( {N{H_3}} \right)}_5}C{O_3}} \right]Cl:$$ Pentaammine carbonatochloridocobaltate(III)
C
$$N{a_3}\left[ {Co{{\left( {N{O_2}} \right)}_6}} \right]:$$ Sodium hexanitrocobaltate(III)
D
$${K_3}\left[ {Cr{{\left( {CN} \right)}_6}} \right]:$$ Potassiumhexacyanidochromate(III)
Answer :
$$\left[ {Co{{\left( {N{H_3}} \right)}_5}C{O_3}} \right]Cl:$$ Pentaammine carbonatochloridocobaltate(III)
22. The hybridisation involved in complex $${\left[ {Ni{{\left( {CN} \right)}_4}} \right]^{2 - }}$$ is ( Atomic number of $$Ni=28$$ )
A
$$ds{p^2}$$
B
$$s{p^3}$$
C
$${d^2}s{p^2}$$
D
$${d^2}s{p^3}$$
Answer :
$$ds{p^2}$$
23.
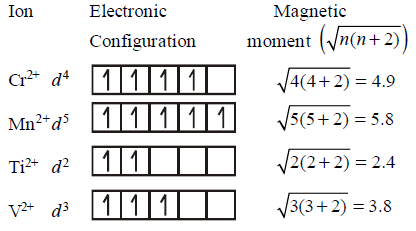
Which of the following paramagnetic ions would exhibit a magnetic moment ( spin only ) of the order of $$5\,BM?$$
$${\text{(}}\,{\text{At}}{\text{. Nos}}{\text{. }}Mn = 25,Cr = 24,V = 23,{\text{ }}Ti = 22\,)$$
A
$$M{n^{2 + }}$$
B
$$T{i^{2 + }}$$
C
$${V^{2 + }}$$
D
$$C{r^{2 + }}$$
Answer :
$$C{r^{2 + }}$$
24. Which of the following isomers will give white precipitate with $$BaC{l_2}$$ solution?
A
$$\left[ {Co{{\left( {N{H_3}} \right)}_5}S{O_4}} \right]Br$$
B
$$\left[ {Co{{\left( {N{H_3}} \right)}_5}Br} \right]S{O_4}$$
C
$$\left[ {Co{{\left( {N{H_3}} \right)}_4}{{\left( {S{O_4}} \right)}_2}} \right]Br$$
D
$$\left[ {Co{{\left( {N{H_3}} \right)}_4}Br\left( {S{O_4}} \right)} \right]$$
Answer :
$$\left[ {Co{{\left( {N{H_3}} \right)}_5}Br} \right]S{O_4}$$
25. Which one of the following has largest number of isomers?
A
$${\left[ {Ir{{\left( {P{R_3}} \right)}_2}H\left( {CO} \right)} \right]^{2 + }}$$
B
$${\left[ {Co{{\left( {N{H_3}} \right)}_5}Cl} \right]^{2 + }}$$
C
$${\left[ {Ru{{\left( {N{H_3}} \right)}_4}C{l_2}} \right]^ + }$$
D
$${\left[ {Co{{\left( {en} \right)}_2}C{l_2}} \right]^ + }\,\left( {{\text{R = alkyl}}\,{\text{group,}}\,{\text{en = ethylenediamine}}} \right)$$
Answer :
$${\left[ {Co{{\left( {en} \right)}_2}C{l_2}} \right]^ + }\,\left( {{\text{R = alkyl}}\,{\text{group,}}\,{\text{en = ethylenediamine}}} \right)$$
26. Hexacyano complexes of metals in their +2 oxidation state are usually yellow while the corresponding hexaaqua compounds are often blue or green. This is so because
A
hexacyano complexes absorb orange or red light thus appear yellow while hexaaqua complexes absorb indigo thus appear yellow
B
hexacyano complexes absorb indigo thus appearing yellow while hexaaqua complexes absorb orange or red light thus appear blue or green
C
hexacyano complexes absorb yellow light while hexaaqua complexes absorb blue light
D
$$C{N^ - }$$ ions are yellow in colour while aqua ions are blue or green in colour
Answer :
hexacyano complexes absorb indigo thus appearing yellow while hexaaqua complexes absorb orange or red light thus appear blue or green
27. Native silver metal forms a water soluble complex with a dilute aqueous solution of $$NaCN$$ in the presence of
A
nitrogen
B
oxygen
C
carbon dioxide
D
argon
Answer :
oxygen
28.
Consider the following isomers.
$$\eqalign{
& \left( {\text{i}} \right)\left[ {Pt{{\left( {N{H_3}} \right)}_4}C{l_2}} \right]B{r_2} \cr
& \left( {{\text{ii}}} \right)\left[ {Pt{{\left( {N{H_3}} \right)}_4}B{r_2}} \right]C{l_2} \cr
& \left( {{\text{iii}}} \right)\left[ {Co{{\left( {N{H_3}} \right)}_4}C{l_2}} \right]N{O_2} \cr} $$
Which of the following observations is correct?
A
(i) will give a pale yellow and (ii) will give a white precipitate with $$AgN{O_3}$$ solution.
B
(iii) will give a white precipitate with $$AgN{O_3}$$ solution.
C
(i), (ii) and (iii) will give white precipitate with $$AgN{O_3}$$ solution.
D
None of the above isomers will give white precipitate with $$AgN{O_3}$$ solution.
Answer :
(i) will give a pale yellow and (ii) will give a white precipitate with $$AgN{O_3}$$ solution.
29. The stabilisation of coordination compounds due to chelation is called the chelate effect. Which of the following is the most stable complex species?
A
$$\left[ {Fe{{\left( {CO} \right)}_5}} \right]$$
B
$${\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{3 - }}$$
C
$${\left[ {Fe{{\left( {{C_2}{O_4}} \right)}_3}} \right]^{3 - }}$$
D
$${\left[ {Fe{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }}$$
Answer :
$${\left[ {Fe{{\left( {{C_2}{O_4}} \right)}_3}} \right]^{3 - }}$$
30. The octahedral complex of a metal ion $${M^{3 + }}$$ with four monodentate ligands $${L_1},{L_2},{L_3}$$ and $${L_4}$$ absorb wavelengths in the region of red, green, yellow and blue, respectively. The increasing order of ligand strength of the four ligands is:
A
$${L_4} < {L_3} < {L_2} < {L_1}$$
B
$${L_1} < {L_3} < {L_2} < {L_4}$$
C
$${L_3} < {L_2} < {L_4} < {L_1}$$
D
$${L_1} < {L_2} < {L_4} < {L_3}$$
Answer :
$${L_1} < {L_3} < {L_2} < {L_4}$$