41. In $$Fe{\left( {CO} \right)_5},$$ the $$Fe-C$$ bond possesses
A
ionic character
B
$$\sigma $$ - character only
C
$$\pi $$ - character
D
both $$\sigma $$ and $$\pi $$ characters
Answer :
both $$\sigma $$ and $$\pi $$ characters
42. The total number of possible isomers of the complex compound $$\left[ {C{u^{II}}{{\left( {N{H_3}} \right)}_4}} \right]\left[ {P{t^{II}}C{l_4}} \right]$$ is
A
3
B
6
C
5
D
4
Answer :
4
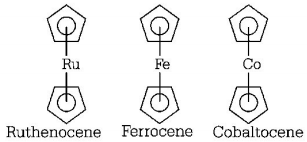
43. An example of a sigma bonded organometallic compound is
A
ruthenocene
B
Grignard’s reagent
C
ferrocene
D
cobaltocene
Answer :
Grignard’s reagent
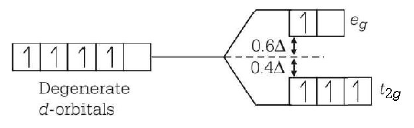
44. Crystal field stabilisation energy for high spin $${d^4}$$ octahedral complex is
A
$$ - 1.8{\Delta _o}$$
B
$$ - 1.6{\Delta _o} + P$$
C
$$ - 1.2{\Delta _o}$$
D
$$ - 0.6{\Delta _o}$$
Answer :
$$ - 0.6{\Delta _o}$$
45. Which among the following will be named as dibromido$$bis$$ (ethylenediamine)chromium(III) bromide?
A
$$\left[ {Cr{{\left( {en} \right)}_2}B{r_2}} \right]Br$$
B
$${\left[ {Cr\left( {en} \right)B{r_4}} \right]^ - }$$
C
$$\left[ {Cr\left( {en} \right)B{r_2}} \right]Br$$
D
$$\left[ {Cr{{\left( {en} \right)}_3}} \right]B{r_3}$$
Answer :
$$\left[ {Cr{{\left( {en} \right)}_2}B{r_2}} \right]Br$$
46. $${\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{4 - }}$$ and $${\left[ {Fe{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$ show different colours in dilute solution because
A
$$C{N^ - }$$ is a strong field ligand and $${H_2}O$$ is a weak field ligand hence magnitude of $$CFSE$$ is different
B
both $$C{N^ - }$$ and $${H_2}O$$ absorb same wavelength of energy
C
complexes of weak field ligands are generally colourless
D
the sizes of $$C{N^ - }$$ and $${H_2}O$$ are different hence their colours are also different
Answer :
$$C{N^ - }$$ is a strong field ligand and $${H_2}O$$ is a weak field ligand hence magnitude of $$CFSE$$ is different
47. $$0.02\,mole$$ of $$\left[ {Co{{\left( {N{H_3}} \right)}_5}Br} \right]C{l_2}$$ and $$0.02\,mole$$ of $$\left[ {Co{{\left( {N{H_3}} \right)}_5}Cl} \right]S{O_4}$$ are present in $$200\,cc$$ of a solution $$X.$$ The number of moles of the precipitates $$Y$$ and $$Z$$ that are formed when the solution $$X$$ is treated with excess silver nitrate and excess barium chloride are respectively
A
0.02, 0.02
B
0.01, 0.02
C
0.02, 0.04
D
0.04, 0.02
Answer :
0.04, 0.02
48.
Which of the following ions are optically active ?
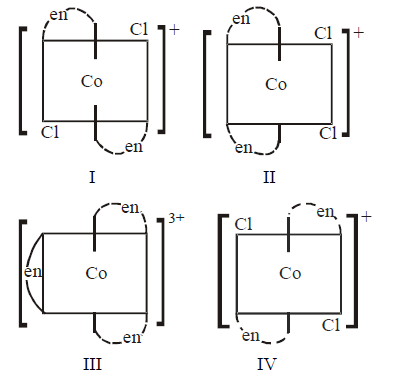
A
I only
B
II only
C
II and III
D
IV only
Answer :
II and III
49.
Which of the following complexes exhibits the highest paramagnetic behaviour ?
where, $$gly =$$ glycine, $$en =$$ ethylenediamine and $$bpy =$$ bipyridyl moities )
$$\left( {{\text{At}}{\text{. no}}{\text{. of}}\,\,Ti = 22,V = 23,} \right.$$ $$\left. {Fe = 26,Co = 27} \right)$$
A
$${\left[ {V{{\left( {gly} \right)}_2}{{\left( {OH} \right)}_2}{{\left( {N{H_3}} \right)}_2}} \right]^ + }$$
B
$${\left[ {Fe\left( {en} \right)\left( {py} \right){{\left( {N{H_3}} \right)}_2}} \right]^{2 + }}$$
C
$${\left[ {Co{{\left( {ox} \right)}_2}{{\left( {OH} \right)}_2}} \right]^ - }$$
D
$${\left[ {Ti{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }}$$
Answer :
$${\left[ {Co{{\left( {ox} \right)}_2}{{\left( {OH} \right)}_2}} \right]^ - }$$
50. $$CuS{O_4} \cdot 5{H_2}O$$ is blue in colour while $$CuS{O_4}$$ is colourless due to
A
presence of strong field ligand in $$CuS{O_4} \cdot 5{H_2}O$$
B
absence of water (ligand), $$d{\text{ - }}d$$ transitions are not possible in $$CuS{O_4}$$
C
anhydrous $$CuS{O_4}$$ undergoes $$d{\text{ - }}d$$ transitions due to crystal field splitting
D
colour is lost due to loss of unpaired electrons
Answer :
absence of water (ligand), $$d{\text{ - }}d$$ transitions are not possible in $$CuS{O_4}$$