71. An aqueous solution of titanium chloride, when subjected to magnetic measurement, measured zero magnetic moment. Assuming the octahedral complex in aqueous solution, the formulae of the complex is :
A
$$\left[ {Ti{{\left( {{H_2}O} \right)}_6}} \right]C{l_2}$$
B
$$\left[ {Ti{{\left( {{H_2}O} \right)}_6}} \right]C{l_4}$$
C
$$\left[ {TiC{l_3}{{\left( {{H_2}O} \right)}_3}} \right]$$
D
$$\left[ {TiC{l_2}{{\left( {{H_2}O} \right)}_4}} \right]$$
Answer :
$$\left[ {Ti{{\left( {{H_2}O} \right)}_6}} \right]C{l_4}$$
72. A coordination complex compound of cobalt has the molecular formula containing five ammonia molecules, one nitro group and two chlorine atoms for one cobalt atom. One mole of this compound produces three mole ions in an aqueous solution. On reacting this solution with excess of $$AgN{O_3}s$$ olution, we get two moles of $$AgCl$$ precipitate. The ionic formula for this complex would be
A
$$\left[ {Co{{\left( {N{H_3}} \right)}_5}\left( {N{O_2}} \right)} \right]C{l_2}$$
B
$$\left[ {Co{{\left( {N{H_3}} \right)}_5}Cl} \right]\left[ {Cl\left( {N{O_2}} \right)} \right]$$
C
$$\left[ {Co{{\left( {N{H_3}} \right)}_4}\left( {N{O_2}} \right)Cl} \right]\left[ {\left( {N{H_3}} \right)Cl} \right]$$
D
$$\left[ {Co{{\left( {N{H_3}} \right)}_5}} \right]\left[ {{{\left( {N{O_2}} \right)}_2}C{l_2}} \right]$$
Answer :
$$\left[ {Co{{\left( {N{H_3}} \right)}_5}\left( {N{O_2}} \right)} \right]C{l_2}$$
73.
In the process of extraction of gold,
Roasted gold ore $$ + C{N^ - } + {H_2}O\mathop \to \limits^{{O_2}} \left[ X \right] + O{H^ - }$$
$$\left[ X \right] + Zn \to \left[ Y \right] + Au$$
Identify the complexes $$\left[ X \right]\,\,{\text{and}}\,\,\left[ Y \right]$$
A
$$X = {\left[ {Au{{\left( {CN} \right)}_2}} \right]^ - },Y = {\left[ {Zn{{\left( {CN} \right)}_4}} \right]^{2 - }}$$
B
$$X = {\left[ {Au{{\left( {CN} \right)}_4}} \right]^{3 - }},Y = {\left[ {Zn{{\left( {CN} \right)}_4}} \right]^{2 - }}$$
C
$$X = {\left[ {Au{{\left( {CN} \right)}_2}} \right]^ - },Y = {\left[ {Zn{{\left( {CN} \right)}_6}} \right]^{4 - }}$$
D
$$X = {\left[ {Au{{\left( {CN} \right)}_4}} \right]^ - },Y = {\left[ {Zn{{\left( {CN} \right)}_4}} \right]^{2 - }}$$
Answer :
$$X = {\left[ {Au{{\left( {CN} \right)}_2}} \right]^ - },Y = {\left[ {Zn{{\left( {CN} \right)}_4}} \right]^{2 - }}$$
74. In which complex, $${d_{{z^2}}}$$ orbital of inner shell is not used in the hybridization of central metal cation
A
$$Fe{\left( {CO} \right)_5}$$
B
$${\left[ {Cu{{\left( {N{H_3}} \right)}_5}} \right]^{2 + }}$$
C
$${\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]^{2 + }}$$
D
$${\left[ {Ir{F_6}} \right]^{3 - }}$$
Answer :
$${\left[ {Cu{{\left( {N{H_3}} \right)}_5}} \right]^{2 + }}$$
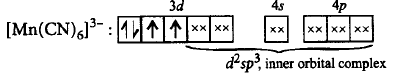
75. Which of the following statements is correct for $${\left[ {Mn{{\left( {CN} \right)}_6}} \right]^{3 - }}$$ according to valence bond theory?
A
$${d^2}s{p^3},$$ inner orbital complex, paramagnetic, 2.82 $$B.M.$$
B
$${d^2}s{p^3},$$ inner orbital complex, diamagnetic, zero magnetic moment.
C
$${d^2}s{p^3},$$ outer orbital complex, paramagnetic, 3.87 $$B.M.$$
D
$$ds{p^2},$$ outer orbital complex, diamagnetic, zero magnetic moment.
Answer :
$${d^2}s{p^3},$$ inner orbital complex, paramagnetic, 2.82 $$B.M.$$
76.
Which one of the following cyano complexes would exhibit the lowest value of paramagnetic behaviour?
$$\left( {{\text{At}}\,{\text{Nos}}:\,Cr = 24,\,Mn = 25,\,Fe = 26,\,Co = 27} \right)$$
A
$${\left[ {Co{{\left( {CN} \right)}_6}} \right]^{3 - }}$$
B
$${\left[ {Fe{{\left( {CN} \right)}_6}} \right]^{3 - }}$$
C
$${\left[ {Mn{{\left( {CN} \right)}_6}} \right]^{3 - }}$$
D
$${\left[ {Cr{{\left( {CN} \right)}_6}} \right]^{3 - }}$$
Answer :
$${\left[ {Co{{\left( {CN} \right)}_6}} \right]^{3 - }}$$
77. The correct energy level diagram for $${\left[ {NiC{l_4}} \right]^{2 - }}$$ is
A
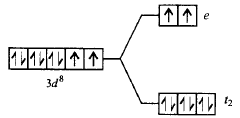

B
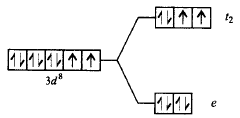

C
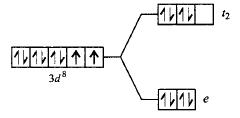

D
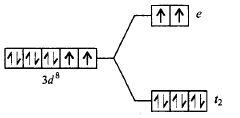

Answer :


78. Which of the following facts about the complex $$\left[ {Cr{{\left( {N{H_3}} \right)}_6}} \right]C{l_3}$$ is wrong?
A
The complex involves $${d^2}s{p^3}$$ hybridisation and is octahedral in shape.
B
The complex is paramagnetic.
C
The complex is an outer orbital complex.
D
The complex gives white precipitate with silver nitrate solution.
Answer :
The complex is an outer orbital complex.
79. Mark the incorrect statement.
A
Inner orbital (low spin) complexes involve $${d^2}s{p^3}$$ hybridisation.
B
Outer orbital (high spin) complexes involve $$s{p^3}{d^2}$$ hybridisation.
C
Tetrahedral complexes generally involve $$ds{p^2}$$ hybridisation.
D
Stereoisomerism involves geometrical and optical isomerism.
Answer :
Tetrahedral complexes generally involve $$ds{p^2}$$ hybridisation.
80. Coordination compounds have great importance in biological systems. In this context which of the following statements is incorrect?
A
Cyanocobalamin is $${B_{12}}$$ and contains cobalt
B
Haemoglobin is the red pigment of blood and contains irons
C
Chlorophylls are green pigments in plants and contain calcium
D
Carboxypeptidase - $$A$$ is an exzyme and contains zinc.
Answer :
Chlorophylls are green pigments in plants and contain calcium