151.
Which group contains coloured ions out of
$$\eqalign{
& \left( {\text{i}} \right)\,C{u^{2 + }} \cr
& \left( {{\text{ii}}} \right)\,T{i^{4 + }} \cr
& \left( {{\text{iii}}} \right)\,C{o^{2 + }} \cr
& \left( {{\text{iv}}} \right)\,F{e^{2 + }} \cr} $$
A
(i), (ii), (iii), (iv)
B
(i), (iii), (iv)
C
(ii), (iii)
D
(i), (ii)
Answer :
(i), (iii), (iv)
152. Which of the following compounds gives red precipitate with $$AgN{O_3}?$$
A
$$KI$$
B
$${K_2}Cr{O_4}$$
C
$$NaBr$$
D
$$NaN{O_3}$$
Answer :
$${K_2}Cr{O_4}$$
153. Which of the following compounds is not coloured?
A
$$N{a_2}\left[ {CuC{l_4}} \right]$$
B
$$N{a_2}\left[ {CdC{l_4}} \right]$$
C
$${K_4}\left[ {Fe{{\left( {CN} \right)}_6}} \right]$$
D
$${K_3}\left[ {Fe{{\left( {CN} \right)}_6}} \right]$$
Answer :
$$N{a_2}\left[ {CdC{l_4}} \right]$$
154. The magnetic moment of a divalent ion in aqueous solution with atomic number 25 is
A
$$5.9\,B.M.$$
B
$$2.9\,B.M.$$
C
$$6.9\,B.M.$$
D
$$9.9\,B.M.$$
Answer :
$$5.9\,B.M.$$
155. Sodium thiosulphate is used in photography because of its
A
reducing behaviour
B
oxidising behaviour
C
complex forming behaviour
D
reaction with light
Answer :
complex forming behaviour
156. Identify the correct structure of dichromate ion.
A
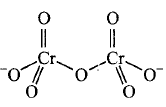

B
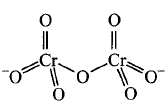

C
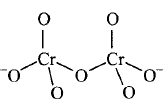

D
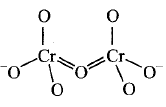

Answer :


157.
For the four successive transition elements $$\left( {Cr,Mn,Fe\,\,{\text{and}}\,\,Co} \right),$$ the stability of + 2 oxidation state will be there in which of the following order?
$$\left( {{\text{At}}{\text{.}}\,{\text{no}}{\text{.}}} \right.\,Cr = 24,Mn = 25,$$ $$\left. {Fe = 26,Co = 27} \right)$$
A
$$Fe > Mn > Co > Cr$$
B
$$Co > Mn > Fe > Cr$$
C
$$Cr > Mn > Co > Fe$$
D
$$Mn > Fe > Cr > Co$$
Answer :
$$Mn > Fe > Cr > Co$$
158. There are 14 elements in actinoid series. Which of the following elements does not belong to this series?
A
$$U$$
B
$$Np$$
C
$$Tm$$
D
$$Fm$$
Answer :
$$Tm$$
159. Stainless steel contains iron and
A
$$Cr + Ni$$
B
$$Cr + Zn$$
C
$$Zn + Pb$$
D
$$Fe + Cr + Ni$$
Answer :
$$Cr + Ni$$
160. When acidified $${K_2}C{r_2}{O_7}$$ solution is added to $$S{n^{2 + }}$$ salts, then $$S{n^{2 + }}$$ changes to
A
$$Sn$$
B
$$S{n^{3 + }}$$
C
$$S{n^{4 + }}$$
D
$$S{n^ + }$$
Answer :
$$S{n^{4 + }}$$