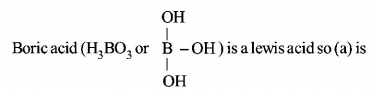1. Which one of the following is the correct statement?
A
Boric acid is a protonic acid
B
Beryllium exhibits coordination number of six
C
Chlorides of both beryllium and aluminium have bridged
chloride structures in solid phase
D
$${B_2}{H_6}2N{H_3}$$ is known as ‘inorganic benzene’
Answer :
Chlorides of both beryllium and aluminium have bridged
chloride structures in solid phase
2. Which of the following are peroxoacids of sulphur?
A
$${H_2}S{O_5}\,\,{\text{and}}\,\,{H_2}{S_2}{O_8}$$
B
$${H_2}S{O_5}\,\,{\text{and}}\,\,{H_2}{S_2}{O_7}$$
C
$${H_2}{S_2}{O_7}\,\,{\text{and}}\,\,{H_2}{S_2}{O_8}$$
D
$${H_2}{S_2}{O_6}\,\,{\text{and}}\,\,{H_2}{S_2}{O_7}$$
Answer :
$${H_2}S{O_5}\,\,{\text{and}}\,\,{H_2}{S_2}{O_8}$$
3. Which of the following bonds will be most polar?
A
$$N—Cl$$
B
$$O—F$$
C
$$N—F$$
D
$$N—N$$
Answer :
$$N—F$$
4. Bauxite ore is generally contaminated with impurity of oxides of two elements $$X$$ and $$Y.$$ Which of the following statement is correct ?
A
$$X$$ is a non-metal and belongs to the third period while $$Y$$ is a metal and belongs to the fourth period.
B
One of two oxides has three-dimensional polymeric structure.
C
Both (A) and (B) are correct.
D
None of the above.
Answer :
Both (A) and (B) are correct.
5. $$AlC{l_3}$$ achieves stability by forming a dimer. In trivalent state the compound is hydrolysed in water. $$AlC{l_3}$$ in acidified aqueous solution forms
A
$$Al{\left( {OH} \right)_3} + HCl$$
B
$${\left[ {Al{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }} + 3C{l^ - }$$
C
$$AlC{l_3} \cdot 2{H_2}O$$
D
$$A{l_2}{O_3} + HCl$$
Answer :
$${\left[ {Al{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }} + 3C{l^ - }$$
6.
In the reaction
$$HN{O_3} + HF \to {H_2}\mathop N\limits^ + {O_3} + {F^ - }$$ base is
A
$$HF$$
B
$$HN{O_3}$$
C
$$HF\,\,{\text{and}}\,\,HN{O_3}$$
D
$${\text{None of these}}$$
Answer :
$$HN{O_3}$$
7. The brown ring test for $$NO_2^ - $$ and $$NO_3^ - $$ is due to the formation of complex ion with a formula
A
$${\left[ {Fe{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
B
$${\left[ {Fe\left( {NO} \right){{\left( {CN} \right)}_5}} \right]^{2 + }}$$
C
$${\left[ {Fe{{\left( {{H_2}O} \right)}_5}NO} \right]^{2 + }}$$
D
$${\left[ {Fe\left( {{H_2}O} \right){{\left( {NO} \right)}_5}} \right]^{2 + }}$$
Answer :
$${\left[ {Fe{{\left( {{H_2}O} \right)}_5}NO} \right]^{2 + }}$$
8.
From the following information
$$X + {H_2}S{O_4} \to Y$$ ( a colourless and irritating gas )
$$Y + {K_2}C{r_2}{O_7} + {H_2}S{O_4} \to $$ ( green coloured solution )
Identify the pair $$X$$ and $$Y.$$
A
$$\bar Cr,HCl$$
B
$$SO_3^{2 - },S{O_2}$$
C
$${S^{2 - }},{H_2}S$$
D
$$CO_3^{2 - },C{O_2}$$
Answer :
$$SO_3^{2 - },S{O_2}$$
9. The structure of diborane $$\left( {{B_2}{H_6}} \right)$$ contains
A
four $$2c - 2e$$ bonds and four $$3c - 2e$$ bonds
B
two $$2c - 2e$$ bonds and two $$3c - 3e$$ bonds
C
two $$2c - 2e$$ bonds and four $$3c - 2e$$ bonds
D
four $$2c - 2e$$ bonds and two $$3c - 2e$$ bonds
Answer :
four $$2c - 2e$$ bonds and two $$3c - 2e$$ bonds
10.
What is the correct observation when $$B{r_2}$$ is treated with $$NaF, NaCl$$ and $$NaI$$ taken in three test-tubes labelled as $$(X), (Y)$$ and $$(Z)?$$

A
$${F_2}$$ is liberated in $$(X)$$ and $$C{l_2}$$ in $$(Y).$$
B
Only $${I_2}$$ is liberated in $$(Z).$$
C
Only $$C{l_2}$$ is liberated in $$(Y).$$
D
Only $${F_2}$$ is liberated in $$(X).$$
Answer :
Only $${I_2}$$ is liberated in $$(Z).$$