91.
Incorrect statement regarding following reactions is :
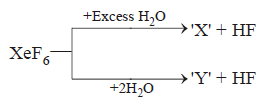
A
$$'X'$$ is explosive
B
$$'Y'$$ is an oxyacid of xenon
C
Both are example of non-redox reaction
D
$$Xe{F_6}$$ can undergo partial hydrolysis.
Answer :
$$'Y'$$ is an oxyacid of xenon
92. Sodium thiosulphate is prepared by
A
reducing $$N{a_2}S{O_4}$$ solution with $${H_2}S$$
B
boiling $$N{a_2}S{O_3}$$ solution with $$S$$ in alkaline medium
C
neutralising $${H_2}{S_2}{O_3}$$ solution with $$\,NaOH$$
D
boiling $$N{a_2}S{O_3}$$ solution with $$S$$ in acidic medium
Answer :
boiling $$N{a_2}S{O_3}$$ solution with $$S$$ in alkaline medium
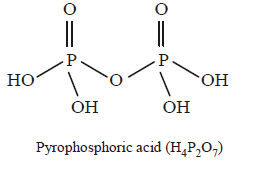
93. The number of $$P–OH$$ bonds and the oxidation state of phosphorus atom in pyrophosphoric acid $$\left( {{H_4}{P_2}{O_7}} \right)$$ respectively are :
A
four and four
B
five and four
C
five and five
D
four and five
Answer :
four and five
94. On heating $$KCl{O_3},$$ we get
A
$$KCl{O_2} + {O_2}$$
B
$$KCl + {O_2}$$
C
$$KCl + {O_3}$$
D
$$KCl + {O_2} + {O_3}$$
Answer :
$$KCl + {O_2}$$
95. Which of the statements given below is incorrect?
A
$$C{l_2}{O_7}$$ is an anhydride of perchloric acid
B
$${O_3}$$ molecule is bent
C
$$ONF$$ is isoelectronic with $$N{O_2}$$
D
$$O{F_2}$$ is an oxide of fluorine
Answer :
$$O{F_2}$$ is an oxide of fluorine
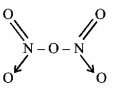
96. The bonds present in $${N_2}{O_5}$$ are :
A
only ionic
B
covalent and coordinate
C
only covalent
D
covalent and ionic
Answer :
covalent and coordinate
97. The role of fluorspar $$\left( {Ca{F_2}} \right)$$ which is added in small quantities in the electrolytic reduction of alumina dissolved in fused cryolite $$\left( {N{a_3}Al{F_6}} \right)$$ is
A
as a catalyst
B
to make the fused mixture very conducting
C
to increase the temperature of the melt.
D
to decrease the rate of oxidation of carbon at the anode.
Answer :
to make the fused mixture very conducting
98. Which of the following is most stable to heat
A
$$HCl$$
B
$$HOCl$$
C
$$HBr$$
D
$$HI$$
Answer :
$$HCl$$
99. Nitrogen forms stable $${N_2}$$ molecule but phosphorus is converted to $${P_4}$$ from $${P_2}$$ because
A
$$p\pi - p\pi $$ bonding is strong in phosphorus
B
$$p\pi - p\pi $$ bonding is weak in phosphorus
C
triple bond is present in phosphorus
D
single $$P-P$$ bond is weaker than $$N-N$$ bond.
Answer :
$$p\pi - p\pi $$ bonding is weak in phosphorus
100. There is no $$S - S$$ bond in :
A
$${S_2}O_4^{2 - }$$
B
$${S_2}O_5^{2 - }$$
C
$${S_2}O_3^{2 - }$$
D
$${S_2}O_7^{2 - }$$
Answer :
$${S_2}O_7^{2 - }$$
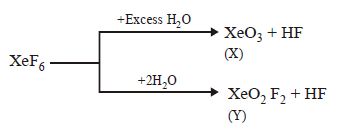

.PNG)