101. Which one of the following orders is not in accordance with the property stated against it?
A
$${F_2} > C{l_2} > B{r_2} > {I_2}$$ Oxidising power
B
$$Hl > HBr > HCl > HF$$ acidic property in water
C
$${F_2} > C{l_2} > B{r_2} > {I_2}$$ Electronegativity
D
$${F_2} > C{l_2} > B{r_2} > {I_2}$$ Bond dissociation energy
Answer :
$${F_2} > C{l_2} > B{r_2} > {I_2}$$ Bond dissociation energy
102. Glass is a
A
super-cooled liquid
B
gel
C
polymeric mixture
D
micro-crystalline solid
Answer :
super-cooled liquid
103. Which of the following is not a use of graphite?
A
For electrodes in batteries.
B
Crucibles made from graphite are used for its inertness to dilute acids and alkalies.
C
For adsorbing poisonous gases.
D
Lubricant at high temperature.
Answer :
For adsorbing poisonous gases.
104. Which of the following is incorrect ?
A
$$M.p$$ of monoclinic sulphur > $$m.p.$$ of rhombic sulphur.
B
Specific gravity of rhombic sulphur > specific gravity of monoclinic sulphur.
C
Monoclinic sulphur is stable below 369 $$K.$$
D
Both rhombic sulphur and monoclinic sulphur have $${S_8}$$ molecules.
Answer :
Monoclinic sulphur is stable below 369 $$K.$$
105.
In the following reactions sequence, \[\left( A \right)+{{N}_{2}}\xrightarrow{\Delta }\left( B \right)\xrightarrow{+{{H}_{2}}O}\] $$\left( C \right) + \left( D \right)$$
white $$ppt.\left( C \right)$$ is formed and gas $$(D)$$ is evolved. White $$ppt.\left( C \right)$$ dissolves in $$NaOH$$ solution, while gas $$(D)$$ gives white fumes in $$HCl.$$ Thus, $$(A)$$ is
A
$$B$$
B
$$Al$$
C
$$Ga$$
D
$$C$$
Answer :
$$Al$$
106.
Correct statements among $$'A'\,{\text{to}}\,'D'\,$$ regarding silicones are:
(A) They are polymers with hydrophobic character.
(B) They are biocompatible.
(C) In general, they have high thermal stability and low dielectric strength.
(D) Usually, they are resistant to oxidation and used as greases.
A
(A), (B),(C) and (D)
B
(A), (B) and (C) only
C
(A) and (B) only
D
(A), (B) and (D) only
Answer :
(A), (B) and (D) only
107. Nitrogen is relatively inactive element because
A
its atom has a stable electronic configuration
B
it has low atomic radius
C
its electronegativity is fairly high
D
dissociation energy of its molecule is fairly high
Answer :
dissociation energy of its molecule is fairly high
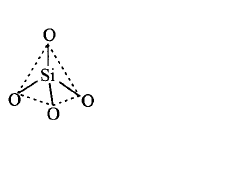
108. Which of the following bonds is shown in silicones?
A
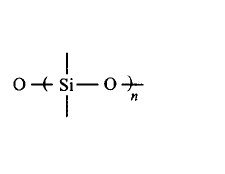
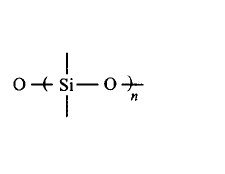
B
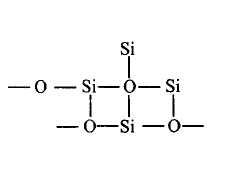
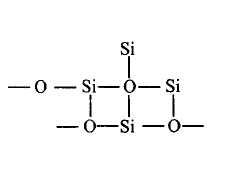
C
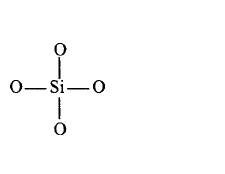

D

Answer :
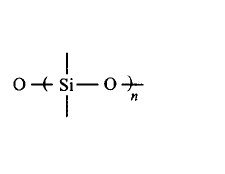
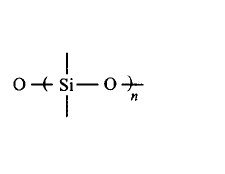
109. Superphosphate of lime is obtained from the reaction of :
A
Bones with gypsum.
B
Calcium phosphate with sulphuric acid.
C
Calcium phosphate with $$HCl.$$
D
Calcium carbonate with phosphoric acid.
Answer :
Calcium phosphate with sulphuric acid.
110.
Match the column I and column II and mark the appropriate choice.
| Column I | Column II | ||
|---|---|---|---|
| a. | $${H_3}P{O_2}$$ | 1. | $$ + 3\,O.S.$$ of $$P$$ |
| b. | $${H_3}{P_3}{O_9}$$ | 2. | Cyclic oxoacid |
| c. | $${H_4}{P_2}{O_6}$$ | 3. | Monobasic acid |
| d. | $${H_3}P{O_3}$$ | 4. | One $$P - P$$ bond |
A
a - 1, b - 3, c - 2, d - 4
B
a - 2, b - 4, c - 3, d - 1
C
a - 3, b - 2, c - 4, d - 1
D
a - 4, b - 1, c - 2, d - 3
Answer :
a - 3, b - 2, c - 4, d - 1