131. On heating with concentrated $$NaOH$$ solution in an inert atmosphere of $$C{O_2},$$ white phosphorus gives a gas. Which of the following statements is incorrect about the gas?
A
It is highly poisonous and has smell like rotten fish.
B
Its solution in water decomposes in the presence of light.
C
It is more basic than $$N{H_3}.$$
D
It is less basic than $$N{H_3}.$$
Answer :
It is more basic than $$N{H_3}.$$
132. Which of the following has the minimum heat of dissociation :
A
$${\left( {C{H_3}} \right)_3}N: \to B{F_3}$$
B
$${\left( {C{H_3}} \right)_3}N: \to B{\left( {C{H_3}} \right)_2}F$$
C
$${\left( {C{H_3}} \right)_3}N: \to B{\left( {C{H_3}} \right)_3}$$
D
$${\left( {C{H_3}} \right)_3}N: \to B\left( {C{H_3}} \right){F_2}$$
Answer :
$${\left( {C{H_3}} \right)_3}N: \to B{\left( {C{H_3}} \right)_3}$$
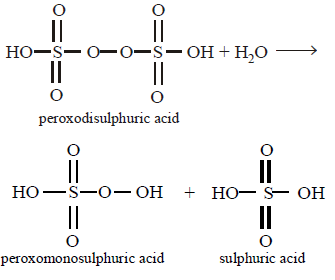
133. Hydrolysis of one mole of peroxodisulphuric acid produces
A
two moles of sulphuric acid.
B
two moles of peroxomonosulphuric acid.
C
one mole of sulphuric acid and one mole of peroxomonosulphuric acid.
D
one mole of sulphuric acid, one mole of peroxomonosulphuric acid and one mole of hydrogen peroxide.
Answer :
one mole of sulphuric acid and one mole of peroxomonosulphuric acid.
134. Oganesson has been synthetically produced by collision of
A
$$Ra\,\,{\text{and}}\,\,Ca$$
B
$$Cf\,\,{\text{and}}\,\,Ca$$
C
$$Cf\,\,{\text{and}}\,\,Cu$$
D
$$Ra\,\,{\text{and}}\,\,He$$
Answer :
$$Cf\,\,{\text{and}}\,\,Ca$$
135. Boron is unable to form $$BF_6^{3 - }$$ ions due to
A
non - availability of $$d$$ - orbitals
B
small size of boron atom
C
non - metallic nature
D
less reactivity towards halogens
Answer :
non - availability of $$d$$ - orbitals
136. Sulphur trioxide is not directly dissolved in water to form sulphuric acid because
A
$$S{O_3}$$ does not react with water to form acid
B
$$S{O_3}$$ gets oxidised to $${H_2}S{O_3}$$ when dissolved in water
C
it results in the formation of dense fog of sulphuric acid which is difficult to condense
D
sulphur trioxide is insoluble in water due to its covalent nature
Answer :
it results in the formation of dense fog of sulphuric acid which is difficult to condense
137. Which of the following oxides is acidic in nature?
A
$${B_2}{O_3}$$
B
$$A{l_2}{O_3}$$
C
$$G{a_2}{O_3}$$
D
$$I{n_2}{O_3}$$
Answer :
$${B_2}{O_3}$$
138. Oxidation number of $$Cl\,\,{\text{in}}\,CaOC{l_2}$$ (bleaching powder) is:
A
zero, since it contains $$C{l_2}$$
B
$$ - 1,$$ since it contains $$Cl$$
C
$$ + 1$$, since it contains $$Cl{O^ - }$$
D
$$ + 1\,{\text{and}}\, - 1$$ since it contains $$Cl{O^ - }\,{\text{and}}\,C{l^ - }$$
Answer :
$$ + 1\,{\text{and}}\, - 1$$ since it contains $$Cl{O^ - }\,{\text{and}}\,C{l^ - }$$
139. What are the products formed in the reaction of xenon hexafluoride with silicon dioxide ?
A
$$XeSi{O_4} + HF$$
B
$$Xe{F_2} + Si{F_4}$$
C
$$XeO{F_4} + Si{F_4}$$
D
$$Xe{O_3} + Si{F_2}$$
Answer :
$$XeO{F_4} + Si{F_4}$$
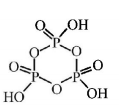
140. The number of $$P\, - \,O\, - P$$ bonds in cyclic metaphosphoric acid is
A
zero
B
two
C
three
D
four
Answer :
three