151. Identify $$X$$ in the reaction : \[X+\underset{\left( \text{Steam} \right)}{\mathop{2{{H}_{2}}O}}\,\xrightarrow{\Delta }X{{O}_{2}}+2{{H}_{2}}\]
A
$$C$$
B
$$Pb$$
C
$$Ge$$
D
$$Sn$$
Answer :
$$Sn$$
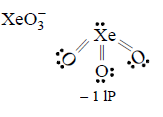
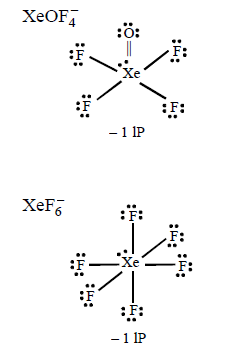
152. Out of $$\left( {\text{i}} \right)Xe{O_3}\left( {{\text{ii}}} \right)XeO{F_4}$$ and $$\left( {{\text{iii}}} \right)Xe{F_6},$$ the molecules having same number of lone pairs on $$Xe$$ are
A
(i) and (ii) only
B
(i) and (iii) only
C
(ii) and (iii) only
D
(i), (ii) and (iii)
Answer :
(i), (ii) and (iii)
153. Which is not the use of orthoboric acid ?
A
As an antiseptic and eye wash.
B
In glass industry.
C
In glazes for pottery.
D
In borax - bead test.
Answer :
In borax - bead test.
154. The increasing order of the first ionization enthalpies of the elements $$B,P,S\,{\text{and}}\,F$$ (Lowest first) is
A
$$B < P < S < F$$
B
$$B < S < P < F$$
C
$$F < S < P < B$$
D
$$P < S < B < F$$
Answer :
$$B < S < P < F$$
155. First ionisation enthalpy of $$Al$$ is lower than that of $$Mg.$$ This is because
A
the size of $$Al$$ is bigger than $$Mg$$
B
ionisation enthalpy decrease in a period from left to right
C
it is easier to remove electron from unpaired $$3{p^1}$$ than from paired $$3{s^2}$$
D
aluminium is a passive metal while magnesium is active metal
Answer :
it is easier to remove electron from unpaired $$3{p^1}$$ than from paired $$3{s^2}$$
156.
Match the column I with column II and mark the appropriate choice.
| Column I | Column II | ||
|---|---|---|---|
| a. | Galena | 1. | Abrasive |
| b. | Diamond | 2. | Metal carbonyls |
| c. | Carbon monoxide | 3. | Hydrides of $$Si$$ |
| d. | Silanes | 4. | An ore of lead |
A
a - 4, b - 2, c - 1, d - 3
B
a - 4, b - 1, c - 2, d - 3
C
a - 2, b - 1, c - 3, d - 4
D
a - 1, b - 2, c - 3, d - 4
Answer :
a - 4, b - 1, c - 2, d - 3
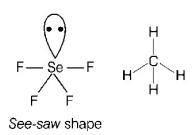
157. Among the following, which one is a wrong statement?
A
$$P{H_5}$$ and $$BiC{l_5}$$ do not exist
B
$$p\pi - d\pi $$ bonds are present in $$S{O_2}$$
C
$$Se{F_4}$$ and $$C{H_4}$$ have same shape
D
$$I_3^ + $$ has bent geometry
Answer :
$$Se{F_4}$$ and $$C{H_4}$$ have same shape
158. Which one of the following compounds is not a protonic acid?
A
$$SO{\left( {OH} \right)_2}$$
B
$$S{O_2}{\left( {OH} \right)_2}$$
C
$$B{\left( {OH} \right)_3}$$
D
$$PO{\left( {OH} \right)_3}$$
Answer :
$$B{\left( {OH} \right)_3}$$
159. Nitrogen is used to fill electric bulbs because
A
it is lighter than air
B
it makes the bulb to glow
C
it does not support combustion
D
it is non toxic
Answer :
it does not support combustion
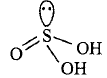
160. The oxyacid of sulphur that contains a lone pair of electrons on sulphur is
A
sulphurous acid
B
sulphuric acid
C
peroxodisulphuric acid
D
pyrosulphuric acid
Answer :
sulphurous acid