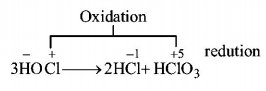
191. What products are expected from the disproportionation reaction of hypochlorous acid?
A
$$HCl\,{\text{and}}\,C{l_2}O$$
B
$$HCl\,{\text{and}}\,HCl{O_3}$$
C
$$HCl{O_3}\,{\text{and}}\,C{l_2}O$$
D
$$HCl{O_2}\,{\text{and}}\,HCl{O_4}$$
Answer :
$$HCl\,{\text{and}}\,HCl{O_3}$$
192. Strong reducing behaviour of $${H_3}P{O_2}$$ is due to
A
low oxidation state of phosphorus
B
presence of two $$- OH$$ groups and one $$P- H$$ bond
C
presence of one $$- OH$$ group and two $$P- H$$ bonds
D
high electron gain enthalpy of phosphorus.
Answer :
presence of one $$- OH$$ group and two $$P- H$$ bonds
193. Which of the following pairs of compounds is isoelectronic and isostructural?
A
$$BeC{l_2},Xe{F_2}$$
B
$$Te{l_2},Xe{F_2}$$
C
$$IBr_2^ - ,Xe{F_2}$$
D
$$l{F_3},Xe{F_2}$$
Answer :
$$IBr_2^ - ,Xe{F_2}$$
194. The geometry of $$XeO{F_4}$$ by $$VSEPR$$ theory is :
A
pentagonal planar
B
octahedral
C
square pyramidal
D
trigonal bipyramidal
Answer :
square pyramidal
195. Which of the following conceivable structures for $$CC{l_4}$$ will have a zero dipole moment ?
A
Square planar
B
Square pyramid (carbon at apex)
C
Irregular tetrahedron
D
None of these
Answer :
None of these
196. The shapes and hybridisation of $$B{F_3}$$ and $$BH_4^ - $$ respectively are
A
$$B{F_3}$$ - Trigonal, $$s{p^2}$$ hybridisation ; $$BH_4^ - $$ - Square planar, $$s{p^3}$$ hybridisation
B
$$B{F_3}$$ - Triangular, $$s{p^3}$$ hybridisation ; $$BH_4^ - $$ - Hexagonal, $$s{p^3}d$$ hybridisation
C
$$B{F_3}$$ - Trigonal, $$s{p^2}$$ hybridisation ; $$BH_4^ - $$ - Tetrahedral, $$s{p^3}$$ hybridisation
D
$$B{F_3}$$ - Tetrahedral, $$s{p^3}$$ hybridisation ; $$BH_4^ - $$ - Tetrahedral, $$s{p^3}$$ hybridisation
Answer :
$$B{F_3}$$ - Trigonal, $$s{p^2}$$ hybridisation ; $$BH_4^ - $$ - Tetrahedral, $$s{p^3}$$ hybridisation
197. Amongst the trihalides of nitrogen which one is least basic?
A
$$N{F_3}$$
B
$$NC{l_{3\,}}$$
C
$$NB{r_3}$$
D
$$N{l_3}$$
Answer :
$$N{F_3}$$
198. $$Al{I_3},$$ when reacts with $$CC{l_4},$$ gives
A
$$AlC{l_3}$$
B
$$C{I_4}$$
C
$$A{l_4}{C_3}$$
D
$${\text{both (A) and (B)}}$$
Answer :
$${\text{both (A) and (B)}}$$
199. Which of the following species has four lone pairs of electrons?
A
$$I$$
B
$${O^ - }$$
C
$$C{l^ - }$$
D
$$He$$
Answer :
$$C{l^ - }$$
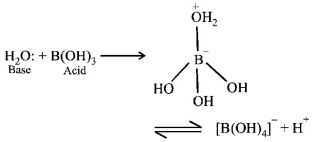
200. $${H_3}B{O_3}$$ is:
A
Monobasic and weak Lewis acid
B
Monobasic and weak Bronsted acid
C
Monobasic and strong Lewis acid
D
Tribasic and weak Bronsted acid
Answer :
Monobasic and weak Lewis acid
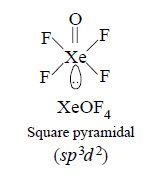

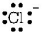 thus, it has four lone pairs of electrons.
thus, it has four lone pairs of electrons.