31.
Complete the given equations :
$$\left( {\text{i}} \right)Cu + 8HN{O_3} \to 3Cu{\left( {N{O_3}} \right)_2}$$ $$ + \underline W + 4{H_2}O$$
$$\left( {{\text{ii}}} \right)4Zn + 10HN{O_3} \to 4Zn{\left( {N{O_3}} \right)_2}$$ $$ + 5{H_2}O + \underline X $$
$$\left( {{\text{iii}}} \right){I_2} + 10HN{O_3} \to \underline Y + 10N{O_2}$$ $$ + 4{H_2}O$$
| $$W$$ | $$X$$ | $$Y$$ | |
|---|---|---|---|
| (a) | $$2N{O_2}$$ | $$NO$$ | $$5HI{O_3}$$ |
| (b) | $$2NO$$ | $${N_2}O$$ | $$2HI{O_3}$$ |
| (c) | $${N_2}$$ | $$N{O_2}$$ | $$HI$$ |
| (d) | $${N_2}O$$ | $$N{O_2}$$ | $$3HI$$ |
A
(a)
B
(b)
C
(c)
D
(d)
Answer :
(b)
32. Which reaction is not feasible?
A
$$2Kl + B{r_2} \to 3KBr + {I_2}$$
B
$$2KBr + {I_2} \to 2Kl + B{r_2}$$
C
$$2KBr + C{l_2} \to 2KCl + B{r_2}$$
D
$$2{H_2}O + 2{F_2} \to 4HF + {O_2}$$
Answer :
$$2KBr + {I_2} \to 2Kl + B{r_2}$$
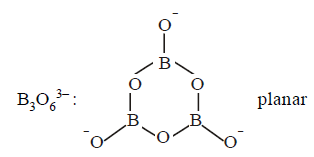
33.
Choose the correct sequence for the geometry of the given molecules
Borazone, Borazole, $${B_3}O_6^{3 - }$$
[ $$'P'$$ stands for planar and $$'N'$$ stands for nonplanar ]
A
$$NP,NP,NP$$
B
$$P,P,NP$$
C
$$NP,P,NP$$
D
$$NP,P,P$$
Answer :
$$NP,P,P$$
34. Which of the following properties of aluminium makes it useful for food packaging ?
A
Good electrical conductivity
B
Good thermal conductivity
C
Low density
D
Non toxicity
Answer :
Low density
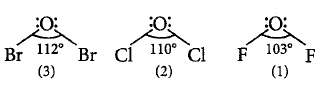
35.
Consider the following oxides :
$$\eqalign{
& \left( {\text{i}} \right)O{F_2} \cr
& \left( {{\text{ii}}} \right)C{l_2}O \cr
& \left( {{\text{iii}}} \right)B{r_2}O \cr} $$
The correct sequence of $$X-O-X$$ bond angle is
A
(i) > (ii) > (iii)
B
(iii) > (ii) > (i)
C
(ii) > (i) > (iii)
D
(i) > (iii) > (ii)
Answer :
(iii) > (ii) > (i)
36. An alkali metal hydride $$\left( {NaH} \right)$$ reacts with diborane in $$'A'$$ to give a tetrahedral compound $$'B'$$ which is extensively used as reducing agent in organic synthesis. The compounds $$'A'$$ and $$'B'$$ respectively are
A
$$C{H_3}COC{H_3}\,\,{\text{and}}\,\,{B_3}{N_3}{H_6}$$
B
$${\left( {{C_2}{H_5}} \right)_2}O\,\,{\text{and}}\,\,NaB{H_4}$$
C
$${C_2}{H_6}\,\,{\text{and}}\,\,{C_2}{H_5}Na$$
D
$${C_6}{H_6}\,\,{\text{and}}\,\,NaB{H_4}$$
Answer :
$${\left( {{C_2}{H_5}} \right)_2}O\,\,{\text{and}}\,\,NaB{H_4}$$
37. Which of the following statements is incorrect?
A
$$S{O_3}$$ is a stronger oxidising agent and more acidic than $$S{O_2}.$$
B
Selenium forms only two oxoacids i.e.,selenous acid $$\left( {{H_2}Se{O_3}} \right)$$ and selenic acid $$\left( {{H_2}Se{O_4}} \right).$$
C
The acidic strength and oxidising power of oxoacids is greater in + 6 oxidation state than in + 4 oxidation state.
D
The thermal stability of oxides of group 16 elements decreases in the order : $$S{O_2} > Se{O_2} > Te{O_2} > Po{O_2}.$$
Answer :
The thermal stability of oxides of group 16 elements decreases in the order : $$S{O_2} > Se{O_2} > Te{O_2} > Po{O_2}.$$
38. Chlorine acts as a bleaching agent only in presence of
A
dryair
B
moisture
C
sunlight
D
pure oxygen
Answer :
moisture
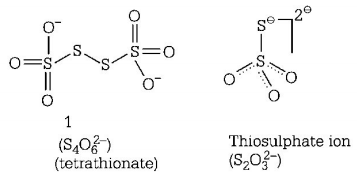
39. In which pair of ions both the species contain $$S—S$$ bond?
A
$${S_2}O_7^{2 - },{S_2}O_3^{2 - }$$
B
$${S_4}O_6^{2 - },{S_2}O_3^{2 - }$$
C
$${S_2}O_7^{2 - },{S_2}O_8^{2 - }$$
D
$${S_4}O_6^{2 - },{S_2}O_7^{2 - }$$
Answer :
$${S_4}O_6^{2 - },{S_2}O_3^{2 - }$$
40. A white precipitate is obtained on hydrolysis of
A
$$PC{l_5}$$
B
$$NC{l_3}$$
C
$$BiC{l_3}$$
D
$$AsC{l_3}$$
Answer :
$$BiC{l_3}$$