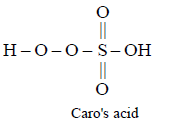51. Which of the following is the wrong statement?
A
Ozone is a paramagnetic gas.
B
The two oxygen-oxygen bond length in ozone are identical.
C
$${O_3}$$ molecule is bent shape.
D
Ozone is violet-black in solid state.
Answer :
Ozone is a paramagnetic gas.
52. The acid which has a peroxy linkage is
A
Sulphurous acid
B
Pyrosulphuric acid
C
Dithionic acid
D
Caro’s acid
Answer :
Caro’s acid
53. Which one of the following is a correct set for $$Si{O_2}?$$
A
Linear acidic
B
Linear,basic
C
Tetrahedral, acidic
D
Angular, basic.
Answer :
Tetrahedral, acidic
54.
Reduction potentials of some ions are given below. Arrange them in decreasing order of oxidising power.
| Ion | $$ClO_4^ - $$ | $$IO_4^ - $$ | $$BrO_4^ - $$ |
|---|---|---|---|
| Reduction potential $${E^ \circ }/V$$ | $${E^ \circ } = 1.19\,V$$ | $${E^ \circ } = 1.65\,V$$ | $${E^ \circ } = 1.74\,V$$ |
A
$$ClO_4^ - > IO_4^ - > BrO_4^ - $$
B
$$IO_4^ - > BrO_4^ - > ClO_4^ - $$
C
$$BrO_4^ - > IO_4^ - > ClO_4^ - $$
D
$$BrO_4^ - > ClO_4^ - > IO_4^ - $$
Answer :
$$BrO_4^ - > IO_4^ - > ClO_4^ - $$
55. Nitrogen forms $${N_2},$$ but phosphorus when form $${P_2}$$ readily converted into $${P_4},$$ reason is
A
triple bond present between phosphorus atom
B
$$p\pi - p\pi $$ bonding is weak
C
$$p\pi - p\pi $$ bonding is strong
D
multiple bond form easily
Answer :
$$p\pi - p\pi $$ bonding is weak
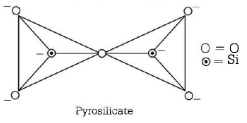
56. Name the type of the structure of silicate in which one oxygen atom of $${\left[ {Si{O_4}} \right]^{4 - }}$$ is shared?
A
Sheet silicate
B
Pyrosilicate
C
Three dimensional silicate
D
Linear chain silicate
Answer :
Pyrosilicate
57. Which of the following statements is true?
A
Silicon exhibits 4 coordination number in its compounds
B
Bond energy of $${F_2}\,$$ is less than $$C{l_2}$$
C
$$Mn\left( {{\text{III}}} \right)$$ oxidation state is more stable than $$Mn\left( {{\text{II}}} \right)$$ in aqueous state
D
Elements of 15th group shows only + 3 and + 5 oxidation states
Answer :
Bond energy of $${F_2}\,$$ is less than $$C{l_2}$$
58. In diborane,
A
four bridged hydrogen atoms and two terminal hydrogen atoms are present
B
two bridged hydrogen atoms and four terminal hydrogen atoms are present
C
three bridged hydrogen atoms and three terminal hydrogen atoms are present
D
there are no bridged hydrogen atoms in diborane, only hydrogen bonds are present.
Answer :
two bridged hydrogen atoms and four terminal hydrogen atoms are present
59. Which is used in the laboratory for fast drying of neutral gases?
A
$${P_2}{O_5}$$
B
$${\text{Anhyd}}{\text{.}}\,CaC{l_2}$$
C
$${\text{Activated charcoal}}$$
D
$$N{a_3}P{O_4}$$
Answer :
$${P_2}{O_5}$$
60. Which of the following increasing order is not correct as mentioned in the property with it?
A
$$HClO < HCl{O_2} < HCl{O_3} < HCl{O_4}$$ ( thermal stability )
B
$$HCl{O_4} < HCl{O_3} < HCl{O_2} < HClO$$ ( oxidising power )
C
$${F^ - } < C{l^ - } < B{r^ - } < {I^ - }$$ ( reducing nature )
D
$$HI{O_4} < ICl < {I_2} < HI$$ ( oxidation number of iodine )
Answer :
$$HI{O_4} < ICl < {I_2} < HI$$ ( oxidation number of iodine )