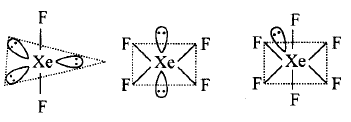
61. In $$Xe{F_2},Xe{F_4}$$ and $$Xe{F_6}$$ the number of lone pairs on $$Xe$$ is respectively
A
2, 3, 1
B
1, 2, 3
C
4, 1, 2
D
3, 2, 1
Answer :
3, 2, 1
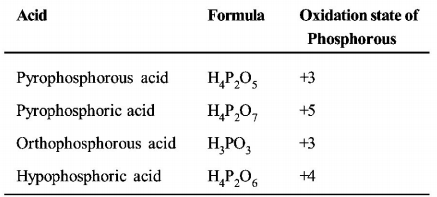
62. The pair in which phosphorous atoms have a formal oxidation state of $$ + 3$$ is :
A
Orthophosphorous and hypophosphoric acids
B
Pyrophosphorous and pyrophosphoric acids
C
Orthophosphorous and pyrophosphorous acids
D
Pyrophosphorous and hypophosphoric acids
Answer :
Orthophosphorous and pyrophosphorous acids
63. Total number of lone pair of electrons in $$XeO{F_4}$$ is
A
0
B
1
C
2
D
3
Answer :
1
64. Which of the following is not true about structure of diamond and graphite?
A
In diamond, each carbon is $$s{p^3}$$ hybridised while in graphite each carbon is $$s{p^2}$$ hybridised.
B
In diamond, carbon atoms are closely packed in crystal lattice while graphite has layer structure.
C
Diamond is a hard substance while graphite is a soft substance.
D
Graphite is thermodynamically very less stable as compared to diamond and is amorphous form of carbon.
Answer :
Graphite is thermodynamically very less stable as compared to diamond and is amorphous form of carbon.
65. The stability of + 1 oxidation state increase in the sequence
A
$$Al < Ga < In < Tl$$
B
$$Tl < In < Ga < Al$$
C
$$In < Tl < Ga < Al$$
D
$$Ga < In < Al < Tl$$
Answer :
$$Al < Ga < In < Tl$$
66. The members of group 14 form tetrahalides of the type, \[M{{X}_{4}}.\] Which of the following halides cannot be readily hydrolysed by water?
A
$$C{X_4}$$
B
$$Si{X_4}$$
C
$$Ge{X_4}$$
D
$$Sn{X_4}$$
Answer :
$$C{X_4}$$
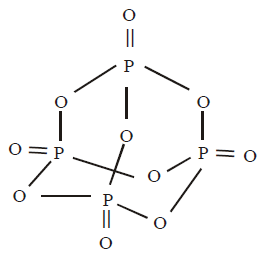
67. Number of sigma bonds in $${P_4}{O_{10}}$$ is
A
6
B
7
C
17
D
16
Answer :
16
68. A greenish yellow gas reacts with an alkali metal hydroxide to form a halate which can be used in fire works safety matches. The gas and halate respectively are
A
$$B{r_2},KBr{O_3}$$
B
$$C{l_2},KCl{O_3}$$
C
$${I_2},NaI{O_3}$$
D
$$C{l_2},NaCl{O_3}$$
Answer :
$$C{l_2},KCl{O_3}$$
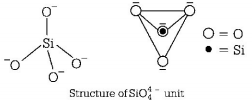
69. The basic structural unit of silicates is
A
$$Si{O^ - }$$
B
$$SiO_4^{4 - }$$
C
$$SiO_3^{2 - }$$
D
$$SiO_4^{2 - }$$
Answer :
$$SiO_4^{4 - }$$
70. Which of the following types of forces bind together the carbon atoms in diamond?
A
lonic
B
Covalent
C
Dipolar
D
van der Waals’
Answer :
Covalent