71.
Complete the following reactions :
$$\eqalign{
& \left( {\text{i}} \right)Si{O_2} + 2NaOH \to X + {H_2}O \cr
& \left( {{\text{ii}}} \right)Si{O_2} + 4HF \to Y + 2{H_2}O \cr} $$
\[\left( \text{iii} \right)Si+2C{{H}_{3}}Cl\xrightarrow[570\,K]{Cu\,\text{powder}}Z\]
| X | Y | Z | |
|---|---|---|---|
| (a) | $$N{a_2}Si{O_3}$$ | $$Si{F_4}$$ | $${\left( {C{H_3}} \right)_2}SiC{l_2}$$ |
| (b) | $${H_2}Si{O_3}$$ | $$Si{F_2}$$ | $$C{H_3}SiC{l_3}$$ |
| (c) | $$N{a_2}Si{O_3}$$ | $${H_2}Si{O_3}$$ | $${\left( {C{H_3}} \right)_3}SiCl$$ |
| (d) | $$N{a_2}Si{O_3}$$ | $${H_2}Si{F_4}$$ | $${\left( {C{H_3}} \right)_2}SiC{l_2}$$ |
A
(a)
B
(b)
C
(c)
D
(d)
Answer :
(a)
72. All members of group 14 when heated in oxygen form oxides. Which of the following is the correct trend of oxides?
A
$$C{O_2}$$ and $$Si{O_2}$$ are acidic.
B
$$CO,GeO,SnO$$ and $$PbO$$ are amphoteric.
C
Monoxides react with haemoglobin to form toxic compounds.
D
All oxides burn with blue flame.
Answer :
$$C{O_2}$$ and $$Si{O_2}$$ are acidic.
73. The aqueous solution of which of the following has maximum $$pH ?$$
A
$$NaClO$$
B
$$NaCl{O_2}$$
C
$$NaCl{O_3}$$
D
$$NaCl{O_4}$$
Answer :
$$NaClO$$
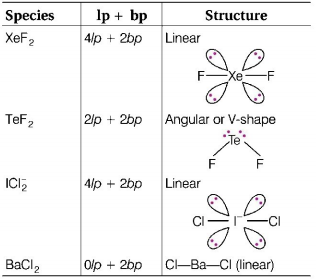
74. $$Xe{F_2}$$ is isostructural with
A
$$Te{F_2}$$
B
$$ICl_2^ - $$
C
$$SbC{l_3}$$
D
$$BaC{l_2}$$
Answer :
$$ICl_2^ - $$
75. In which of the following, a salt of the type $$KM{O_2}$$ is obtained ?
A
$${B_2}{H_6} + KOH\left( {aq} \right) \to $$
B
$$Al + KOH\left( {aq} \right) \to $$
C
$${\text{Both}}$$
D
$${\text{None}}$$
Answer :
$${\text{Both}}$$
76. Bromine can be liberated from potassium bromide solution by the action of
A
lodine solution
B
Chlorine water
C
Sodium chloride
D
Potassium iodide
Answer :
Chlorine water
77. Which one of the following molecular hydrides acts as a Lewis acid?
A
$$N{H_3}$$
B
$${H_2}O$$
C
$${B_2}{H_6}$$
D
$$C{H_4}$$
Answer :
$${B_2}{H_6}$$
78. Ammonia is used in detection of $$C{u^{2 + }}$$ ion because
A
aqueous solution of $$N{H_3}$$ reacts with $$C{u^{2 + }}$$ ion to form deep blue coloured complex
B
$$N{H_3}$$ reacts with $$C{u^{2 + }}$$ ion to give blue precipitate of $$CuO$$
C
aqueous solution of $$N{H_3}$$ reacts with $$C{u^{2 + }}$$ ion to form white coloured complex
D
$$N{H_3}$$ reacts with $$C{u^{2 + }}$$ ion to give green precipitate
Answer :
aqueous solution of $$N{H_3}$$ reacts with $$C{u^{2 + }}$$ ion to form deep blue coloured complex
79. Borax is not used
A
as a styptic to stop bleeding
B
in making enamel and pottery glazes
C
as a flux in soldering
D
in making optical glasses
Answer :
as a styptic to stop bleeding
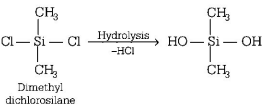
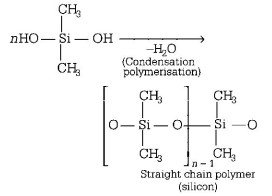
80. The straight chain polymer is formed by
A
hydrolysis of $${\left( {C{H_3}} \right)_3}SiCl$$ followed by condensation polymerisation
B
hydrolysis of $$C{H_3}SiC{l_3}$$ followed by condensation polymerisation
C
hydrolysis of $${\left( {C{H_3}} \right)_4}Si$$ by addition polymerisation
D
hydrolysis of $${\left( {C{H_3}} \right)_2}SiC{l_2}$$ followed by condensation polymerisation
Answer :
hydrolysis of $${\left( {C{H_3}} \right)_2}SiC{l_2}$$ followed by condensation polymerisation